Introduction
Protein-protein interactions (PPIs) control variety of biological phenomena including development, cell to cell interactions and metabolic processes. The PPIs can be classified into different groups, depending upon their functional and structural properties. Depending upon their persistence, they may be termed as permanent or transient, as characterized by their interaction surface, they may be considered as heterooligomeric or homooligomeric based on their stability, and they may be called as obligate or nonobligate A blend of these three pairs may develop a protein-protein interaction. For example, a permanent interaction of the protein may be able to form a stable protein complex while on the other hand a transient interaction among the proteins may form a signaling pathway
To perform the function in a living cell, proteins rarely act as isolated species instead over 80% of the proteins perform their functions in groups . The function of an unidentified protein can be suggested by its interactions with a protein of known function. The thorough study of PPIs is also important to demonstrate the molecular mechanism of cellular processes of proteins.
The momentous properties of PPIs are
(a) the kinetic properties of the enzymes can be modified by PPIs;
(b) PPIs can allow substrate channeling;
(c) they can create a new binding site for the small molecules;
(d) PPIs can suppress or activate a protein;
(e) PPIs can perform regulatory role in downstream or upstream regulation of the protein; and (f) they can also alter the specificity of binding of the protein for its particular substrate by changing its interactions.
The proteins that have many interactions include transcription factors and enzymes. Though, PPIs encompass heterogeneous procedures mostly and the possibility of their regulation is enormous. Various interactions and the outcome of these interactions are needed to identify the better understanding of PPIs inside the cell. By using methods like mass spectrometry, protein chip technology, phage display, and two hybrid system, PPIs data have been increased in recent years. These experimental resources are useful for constructing comprehensive PPI networks. But, day by day the increase in the amount of data on protein-protein interactions is becoming a challenge for validation in the laboratory. To understand the functions of unexplored protein by using computational approaches is necessary nowadays.
2. Protein-protein interaction assays
Protein-protein interaction (PPI) assays can be classified into three broad categories, i.e.,
- In vivo
- In vitro
- In silico
(1) The in vivo techniques apply the whole procedure on the living cell or organism itself.
(2) In vitro methods require the whole procedure completed outside the cell in a controlled environment in a laboratory, i.e., affinity chromatography, tandem affinity purification (TAP), protein fragment complementation, X-ray crystallography, co-immunoprecipitation, phage display, nuclear magnetic resonance, spectroscopy, and protein arrays.
(3) The techniques that are performed by using computers or computer simulations are called in silico techniques. The sequence and structure-based approaches, gene fusion, chromosome proximity, gene expression-based approaches, domain pair-based approach, in silico two hybrid approaches, phylogenetic profile, and phylogenetic tree are some approaches which are based on in silico methods
2.1. In vivo techniques for the prediction of protein-protein interactions
The in vivo technique used to study PPIs is yeast two hybrid (Y2H) method. The two protein domains are involved in the Y2H assay.
First domain is the DNA binding domain which helps in binding the DNA
The second one is activation domain that is involved in activation of the transcription of the specific DNA.
These two domains are required for the transcription of a particular reporter gene. The interacting proteins that are involved in the Y2H assays must be present in the close vicinity or inside the nucleus because these proteins have the capability to activate reporter gene and the proteins that are not present in nucleus do not have the ability to activate reporter genes. Some other techniques being used are fluorescence resonance energy transfer (FRET), biomolecular fluorescence complementation (BiFC), and bioluminescence resonance energy transfer (BRET)
2.2. In vitro techniques for the prediction of protein-protein interactions
To learn PPIs in the inherent environment of the cell, a technique called TAP tagging was developed. TAP tagging method was first used to analyze the yeast interactome in a high throughput way. TAP tagging involves two steps, first is double tagging of the protein of interest and second is two-step process of purification After the process, the proteins that remain attached with the target protein can be studied by using SDS-PAGE and then mass spectrometry analysis is performed to confirm the PPI partner of the protein of interest. TAP tagging used in combination with mass spectrometry which can identify both protein complexes and protein interactions.
Affinity chromatography is also used to study PPIs in vitro. It is very sensitive technique and can identify even the weakest interactions among the proteins. Though, it generates many of the false positive results because of the great specificity of the proteins. Therefore, studies of protein interactions cannot depend only on affinity chromatography. So, other techniques are needed in combination with affinity chromatography to further confirm the results generated. The affinity chromatography is often combined with mass spectroscopy and SDS-PAGE to produce more convincing results.
Co-immunoprecipitation is another in vitro technique that is used for the confirmation of PPIs by using the complete cell extract wherever proteins are present in a complicated blend of cellular machinery and in their natural form that is essential for the significant interactions of proteins.
Protein arrays are also being used to study PPIs. A piece of glass is used in which different protein molecules are attached in an organized fashion. Protein microarray analysis gained marvelous importance to do high throughput analysis by running many of the parallel analysis in an automated procedure. The PPIs can be studied by using another proteomics method known as protein fragment complementation assay (PCA). It consists of a family of assays that can be used to identify the proteins of any molecular weight and it provides very simple and straight conducts to determine PPIs in living cells, in vitro, and multicellular organisms.
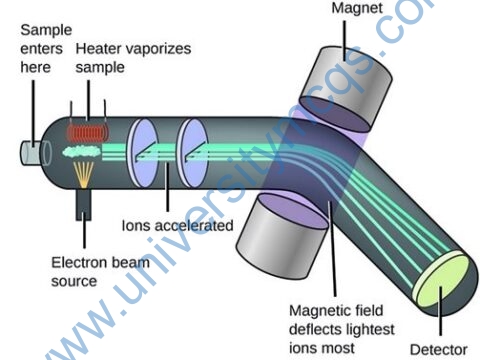
Mass spectroscopy can also be used to determine protein-protein interactions. There are two ways to identify PPIs by using mass spectroscopy shotgun proteomics and peptide finger printing. To analyze complicated mixtures, shotgun proteomics is the most suitable technique while in the peptide finger printing, SDS-PAGE is used to separate the eluted complex. X-ray crystallography can also be used to determine PPIs in vitro. It is a type of microscopy with very high resolution that is used for the identification of proteins at atomic level and it is helpful for functional analysis of proteins.
The analysis of PPIs can also be done by using nuclear magnetic resonance (NMR) spectroscopy. In NMR spectroscopy, the magnetically active nuclei that are surrounded by a strong magnetic field engross electromagnetic radiations at distinguishing frequencies that are monitored by the chemical surroundings.
2.3. In silico techniques to predict protein-protein interactions
Many of the in vivo and in vitro techniques generate a large amount of data that is helpful in the development of software and tools for the identification of PPIs among various proteins that are found in many different combinations.
Protein interactions database: Protein interactions are collected together in specialized biological databases Databases can be subdivided into
- Primary databases
- Meta-databases
- Prediction databases
Primary databases – published PPIs proven to exist via small-scale large- scale experimental methods. Eg: DIP, Biomolecular Interaction Network, BIND, BioGRID), HPRD
Meta-database – Primary and original data Eg: APID, The Microbial, MPIDB, and PINA , and GPS-Prot etc.
Prediction Databases – predicted using several techniques Eg: Human Protein–Protein Interaction Prediction Database (PIPs), I2D, STRING, and Unified Human Interactive (UniHI).
- BIND (Biomolecular Interaction Network Database) • http://bind.ca • A free, open-source database for archiving and exchanging molecular assembly information. • The database contains – Interactions – Molecular complexes – Pathways
- PPI methodologies have been developed in yeast-methods are sometimes not suitable for plant systems Proteomic approaches still challenging International Plant Proteomics Organization (www.inppo.com), global initiative to develop and improve connections between plant proteomics researchers and related fields Conclusions