PROTEINS AND PROTEIN METABOLISM MCQS, Introduction to Biochemistry
1. All proteins contain the
(A) Same 20 amino acids
(B) Different amino acids
(C) 300 Amino acids occurring in nature
(D) Only a few amino acids
2. Proteins contain
(A) Only L- á – amino acids
(B) Only D-amino acids
(C) DL-Amino acids
(D) Both (A) and (B)
3. The optically inactive amino acid is
(A) Glycine (B) Serine
(C) Threonine (D) Valine
4. At neutral pH, a mixture of amino acids in solution would be predominantly:
(A) Dipolar ions
(B) Nonpolar molecules
(C) Positive and monovalent
(D) Hydrophobic
5. The true statement about solutions of amino acids at physiological pH is
(A) All amino acids contain both positive and
negative charges
(B) All amino acids contain positively charged
side chains
(C) Some amino acids contain only positive
charge
(D) All amino acids contain negatively charged
side chains
6. pH (isoelectric pH) of alanine is
(A) 6.02 (B) 6.6
(C) 6.8 (D) 7.2
7. Since the pK values for aspartic acid are 2.0, 3.9 and 10.0, it follows that the isoelectric (pH) is
(A) 3.0 (B) 3.9
(C) 5.9 (D) 6.0
8. Sulphur containing amino acid is
(A) Methionine (B) Leucine
(C) Valine (D) Asparagine
9. An example of sulphur containing amino acid is
(A) 2-Amino-3-mercaptopropanoic acid
(B) 2-Amino-3-methylbutanoic acid
(C) 2-Amino-3-hydroxypropanoic acid
(D) Amino acetic acid
10. All the following are sulphur containing amino acids found in proteins except
(A) Cysteine (B) Cystine
(C) Methionine (D) Threonine
11. An aromatic amino acid is
(A) Lysine (B) Tyrosine
(C) Taurine (D) Arginine
12. The functions of plasma albumin are
(A) Osmosis (B) Transport
(C) Immunity (D) both (A )and (B)
13. Amino acid with side chain containing
basic groups is
(A) 2-Amino 5-guanidovaleric acid
(B) 2-Pyrrolidine carboxylic acid
(C) 2-Amino 3-mercaptopropanoic acid
(D) 2-Amino propanoic acid
14. An example of á-amino acid not present
in proteins but essential in mammalian
metabolism is
(A) 3-Amino 3-hydroxypropanoic acid
(B) 2-Amino 3-hydroxybutanoic acid
(C) 2-Amino 4-mercaptobutanoic acid
(D) 2-Amino 3-mercaptopropanoic acid
15. An essential amino acid in man is
(A) Aspartate (B) Tyrosine
(C) Methionine (D) Serine
16. Non essential amino acids
(A) Are not components of tissue proteins
(B) May be synthesized in the body from essential
amino acids
(C) Have no role in the metabolism
(D) May be synthesized in the body in diseased
states
17. Which one of the following is semiessential
amino acid for humans?
(A) Valine (B) Arginine
(C) Lysine (D) Tyrosine
18. An example of polar amino acid is
(A) Alanine (B) Leucine
(C) Arginine (D) Valine
19. The amino acid with a nonpolar side chain
is
(A) Serine (B) Valine
(C) Asparagine (D) Threonine
20. A ketogenic amino acid is
(A) Valine (B) Cysteine
(C) Leucine (D) Threonine
21. An amino acid that does not form an á–
helix is
(A) Valine (B) Proline
(C) Tyrosine (D) Tryptophan
22. An amino acid not found in proteins is
(A) â-Alanine (B) Proline
(C) Lysine (D) Histidine
23. In mammalian tissues serine can be a
biosynthetic precursor of
(A) Methionine (B) Glycine
(C) Tryptophan (D) Phenylalanine
24. A vasodilating compound is produced by
the decarboxylation of the amino acid:
(A) Arginine (B) Aspartic acid
(C) Glutamine (D) Histidine
25. Biuret reaction is specific for
(A) –CONH-linkages (B) –CSNH2 group
(C) –(NH)NH2 group (D) All of these
26. Sakaguchi’s reaction is specific for
(A) Tyrosine (B) Proline
(C) Arginine (D) Cysteine
27. Million-Nasse’s reaction is specific for the
amino acid:
(A) Tryptophan (B) Tyrosine
(C) Phenylalanine (D) Arginine
28. Ninhydrin with evolution of CO2 forms a
blue complex with
(A) Peptide bond (B) á -Amino acids
(C) Serotonin (D) Histamine
29. The most of the ultraviolet absorption of
proteins above 240 nm is due to their
content of
(A) Tryptophan (B) Aspartate
(C) Glutamate (D) Alanine
30. Which of the following is a dipeptide?
(A) Anserine (B) Glutathione
(C) Glucagon (D) â -Lipoprotein
31. Which of the following is a tripeptide?
(A) Anserine (B) Oxytocin
(C) Glutathione (D) Kallidin
32. A peptide which acts as potent smooth
muscle hypotensive agent is
(A) Glutathione (B) Bradykinin
(C) Tryocidine (D) Gramicidin-s
33. A tripeptide functioning as an important
reducing agent in the tissues is
(A) Bradykinin (B) Kallidin
(C) Tyrocidin (D) Glutathione
34. An example of metalloprotein is
(A) Casein (B) Ceruloplasmin
(C) Gelatin (D) Salmine
35. Carbonic anhydrase is an example of
(A) Lipoprotein (B) Phosphoprotein
(C) Metalloprotein (D) Chromoprotein
36. An example of chromoprotein is
(A) Hemoglobin (B) Sturine
(C) Nuclein (D) Gliadin
37. An example of scleroprotein is
(A) Zein (B) Keratin
(C) Glutenin (D) Ovoglobulin
38. Casein, the milk protein is
(A) Nucleoprotein (B) Chromoprotein
(C) Phosphoprotein (D) Glycoprotein
39. An example of phosphoprotein present
in egg yolk is
(A) Ovoalbumin (B) Ovoglobulin
(C) Ovovitellin (D) Avidin
40. A simple protein found in the nucleoproteins
of the sperm is
(A) Prolamine (B) Protamine
(C) Glutelin (D) Globulin
41. Histones are
(A) Identical to protamine
(B) Proteins rich in lysine and arginine
(C) Proteins with high molecular weight
(D) Insoluble in water and very dilute acids
42. The protein present in hair is
(A) Keratin (B) Elastin
(C) Myosin (D) Tropocollagen
43. The amino acid from which synthesis of
the protein of hair keratin takes place is
(A) Alanine (B) Methionine
(C) Proline (D) Hydroxyproline
44. In one molecule of albumin the number
of amino acids is
(A) 510 (B) 590
(C) 610 (D) 650
45. Plasma proteins which contain more than
4% hexosamine are
(A) Microglobulins (B) Glycoproteins
(C) Mucoproteins (D) Orosomucoids
46. After releasing O2 at the tissues,
hemoglobin transports
(A) CO2 and protons to the lungs
(B) O2 to the lungs
(C) CO2 and protons to the tissue
(D) Nutrients
47. Ehlers-Danlos syndrome characterized by
hypermobile joints and skin abnormalities
is due to
(A) Abnormality in gene for procollagen
(B) Deficiency of lysyl oxidase
(C) Deficiency of prolyl hydroxylase
(D) Deficiency of lysyl hydroxylase
48. Proteins are soluble in
(A) Anhydrous acetone(B) Aqueous alcohol
(C) Anhydrous alcohol (D) Benzene
49. A cereal protein soluble in 70% alcohol
but insoluble in water or salt solution is
(A) Glutelin (B) Protamine
(C) Albumin (D) Gliadin
50. Many globular proteins are stable in
solution inspite they lack in
(A) Disulphide bonds (B) Hydrogen bonds
(C) Salt bonds (D) Non polar bonds
51. The hydrogen bonds between peptide
linkages of a protein molecules are interfered
by
(A) Guanidine (B) Uric acid
(C) Oxalic acid (D) Salicylic acid
52. Globular proteins have completely folded,
coiled polypeptide chain and the axial
ratio (ratio of length to breadth) is
(A) Less than 10 and generally not greater than
3–4
(B) Generally 10
(C) Greater than 10 and generally 20
(D) Greater than 10
53. Fibrous proteins have axial ratio
(A) Less than 10
(B) Less than 10 and generally not greater than
3–4
(C) Generally 10
(D) Greater than 10
54. Each turn of α-helix contains the amino
acid residues (number):
(A) 3.6 (B) 3.0
(C) 4.2 (D) 4.5
55. Distance traveled per turn of á−helix in
nm is
(A) 0.53 (B) 0.54
(C) 0.44 (D) 0.48
56. Along the á-helix each amino acid residue
advances in nm by
(A) 0.15 (B) 0.10
(C) 0.12 (D) 0.20
57. The number of helices present in a
collagen molecule is
(A) 1 (B) 2
(C) 3 (D) 4
58. In proteins the á-helix and â-pleated sheet
are examples of
(A) Primary structure (B) Secondary structure
(C) Tertiary structure (D) Quaternary structure
59. The a-helix of proteins is
(A) A pleated structure
(B) Made periodic by disulphide bridges
(C) A non-periodic structure
(D) Stabilised by hydrogen bonds between NH
and CO groups of the main chain
60. At the lowest energy level á-helix of
polypeptide chain is stabilised
(A) By hydrogen bonds formed between the H of
peptide N and the carbonyl O of the residue
(B) Disulphide bonds
(C) Non polar bonds
(D) Ester bonds
61. Both á-helix and â-pleated sheet conformation
of proteins were proposed by
(A) Watson and Crick
(B) Pauling and Corey
(C) Waugh and King
(D) Y.S.Rao
62. The primary structure of fibroin, the
principal protein of silk worm fibres
consists almost entirely of
(A) Glycine (B) Aspartate
(C) Keratin (D) Tryptophan
63. Tertiary structure of a protein describes
(A) The order of amino acids
(B) Location of disulphide bonds
(C) Loop regions of proteins
(D) The ways of protein folding
64. In a protein molecule the disulphide bond
is not broken by
(A) Reduction
(B) Oxidation
(C) Denaturation
(D) X-ray diffraction
65. The technique for purification of proteins
that can be made specific for a given
protein is
(A) Gel filtration chromotography
(B) Ion exchange chromatography
(C) Electrophoresis
(D) Affinity chromatography
66. Denaturation of proteins results in
(A) Disruption of primary structure
(B) Breakdown of peptide bonds
(C) Destruction of hydrogen bonds
(D) Irreversible changes in the molecule
67. Ceruloplasmin is
(A) á1-globulin (B) á2-globulin
(C) â-globulin (D) None of these
68. The lipoprotein with the fastest electrophoretic
mobility and the lowest triglyceride
content is
(A) Chylomicron (B) VLDL
(C) IDL (D) HDL
69. The lipoprotein associated with activation
of LCAT is
(A) HDL (B) LDL
(C) VLDL (D) IDL
70. The apolipoprotein which acts as activator
of LCAT is
(A) A-I (B) A-IV
(C) C-II (D) D
71. The apolipoprotein which acts as actiator
of extrahepatic lipoprotein is
(A) Apo-A (B) Apo-B
(C) Apo-C (D) Apo-D
72. The apolipoprotein which forms the
integral component of chylomicron is
(A) B-100 (B) B-48
(C) C (D) D
73. The apolipoprotein which from the
integral component of VLDL is
(A) B-100 (B) B-48
(C) A (D) D
74. The apolipoprotein which acts as ligand
for LDL receptor is
(A) B-48 (B) B-100
(C) A (D) C
75. Serum LDL has been found to be increased
in
(A) Obstructive jaundice
(B) Hepatic jaundice
(C) Hemolytic jaundice
(D) Malabsorption syndrome
76. A lipoprotein associated with high
incidence of coronary atherosclerosis is
(A) LDL (B) VLDL
(C) IDL (D) HDL
77. A lipoprotein inversely related to the
incidence of coronary artherosclerosis is
(A) VLDL (B) IDL
(C) LDL (D) HDL
78. The primary biochemical lesion in homozygote
with familial hypercholesterolemia
(type IIa) is
(A) Loss of feed back inhibition of HMG
reductase
(B) Loss of apolipoprotein B
(C) Increased production of LDL from VLDL
(D) Functional deficiency of plasma membrane
receptors for LDL
79. In abetalipoproteinemia, the biochemical
defect is in
(A) Apo-B synthesis
(B) Lipprotein lipase activity
(C) Cholesterol ester hydrolase
(D) LCAT activity
80. Familial hypertriaacylglycerolemia is
associated with
(A) Over production of VLDL
(B) Increased LDL concentration
(C) Increased HDL concentration
(D) Slow clearance of chylomicrons
81. For synthesis of prostaglandins, the
essential fatty acids give rise to a fatty
acid containing
(A) 12 carbon atoms (B) 16 carbon atoms
(C) 20 carbon atoms (D) 24 carbon atoms
82. All active prostaglandins have at least one
double bond between positions
(A) 7 and 8 (B) 10 and 11
(C) 13 and 14 (D) 16 and 17
83. Normal range of plasma total phospholipids
is
(A) 0.2–0.6 mmol/L (B) 0.9–2.0 mmol/L
(C) 1.8–5.8 mmol/L (D) 2.8–5.3 mmol/L
84. HDL2 have the density in the range of
(A) 1.006–1.019 (B) 1.019–1.032
(C) 1.032–1.063 (D) 1.063–1.125
85. â-lipoproteins have the density in the
range of
(A) 0.95–1.006 (B) 1.006–1.019
(C) 1.019–1.063 (D) 1.063–1.125
86. IDL have the density in the range of
(A) 0.95–1.006 (B) 1.006–1.019
(C) 1.019–1.032 (D) 1.032–1.163
87. Aspirin inhibits the activity of the enzyme:
(A) Lipoxygenase (B) Cyclooxygenase
(C) Phospholipae A1 (D) Phospholipase A2
88. A ’suicide enzyme’ is
(A) Cycloxygenase (B) Lipooxygenase
(C) Phospholipase A1 (D) Phospholipase A2
89. In adipose tissue prostaglandins
decrease
(A) Lipogenesis (B) Lipolysis
(C) Gluconeogenesis (D) Glycogenolysis
90 The optimal pH for the enzyme pepsin is
(A) 1.0–2.0 (B) 4.0–5.0
(C) 5.2–6.0 (D) 5.8–6.2
91. Pepsinogen is converted to active pepsin
by
(A) HCl (B) Bile salts
(C) Ca++ (D) Enterokinase
92. The optimal pH for the enzyme rennin is
(A) 2.0 (B) 4.0
(C) 8.0 (D) 6.0
93. The optimal pH for the enzyme trypsin is
(A) 1.0–2.0 (B) 2.0–4.0
(C) 5.2–6.2 (D) 5.8–6.2
94. The optimal pH for the enzyme chymotrypsin
is
(A) 2.0 (B) 4.0
(C) 6.0 (D) 8.0
95 Trypsinogen is converted to active trypsin
by
(A) Enterokinase (B) Bile salts
(C) HCl (D) Mg++
96 Pepsin acts on denatured proteins to
produce
(A) Proteoses and peptones
(B) Polypeptides
(C) Peptides
(D) Dipeptides
97. Renin converts casein to paracasein in
presence of
(A) Ca++ (B) Mg++
(C) Na+ (D) K+
98. An expopeptidase is
(A) Trypsin (B) Chymotrypsin
(C) Elastase (D) Elastase
99. The enzyme trypsin is specific for peptide
bonds of
(A) Basic amino acids
(B) Acidic amino acids
(C) Aromatic amino acids
(D) Next to small amino acid residues
100. Chymotrypsin is specific for peptide bonds
containing
(A) Uncharged amino acid residues
(B) Acidic amino acids
(C) Basic amino acid
(D) Small amino acid residues
101. The end product of protein digestion in
G.I.T. is
(A) Dipeptide (B) Tripeptide
(C) Polypeptide (D) Amino acid
102. Natural L-isomers of amino acids are
absorbed from intestine by
(A) Passive diffusion (B) Simple diffusion
(C) Faciliated diffusion(D) Active process
103. Abnormalities of blood clotting are
(A) Haemophilia (B) Christmas disease
(C) Gout (D) Both (A) and (B)
104. An important reaction for the synthesis
of amino acid from carbohydrate
intermediates is transamination which
requires the cofactor:
(A) Thiamin (B) Riboflavin
(C) Niacin (D) Pyridoxal phosphate
105. The main sites for oxidative deamination
are
(A) Liver and kidney
(B) Skin and pancreas
(C) Intestine and mammary gland
(D) Lung and spleen
106. A positive nitrogen balance occurs
(A) In growing infant
(B) Following surgery
(C) In advanced cancer
(D) In kwashiorkar
107. The main site of urea synthesis in mammals
is
(A) Liver (B) Skin
(C) Intestine (D) Kidney
108. The enzymes of urea synthesis are found
in
(A) Mitochondria only
(B) Cytosol only
(C) Both mitochondria and cytosol
(D) Nucleus
109. The number of ATP required for urea
synthesis is
(A) 0 (B) 1
(C) 2 (D) 3
110. Most of the ammonia released from L-á–
amino acids reflects the coupled action of
transaminase and
(A) L-glutamate dehydrogenase
(B) L-amino acid oxidase
(C) Histidase
(D) Serine dehydratase
111. In urea synthesis, the amino acid functioning
solely as an enzyme activator:
(A) N-acetyl glutamate (B) Ornithine
(C) Citrulline (D) Arginine
112. The enzyme carbamoyl phosphate
synthetase requires
(A) Mg++ (B) Ca++
(C) Na+ (D) K+
113. Control of urea cycle involves the enzyme:
(A) Carbamoyl phosphate synthetase
(B) Ornithine transcarbamoylase
(C) Argininosuccinase
(D) Arginase
114. Transfer of the carbamoyl moiety of
carbamoyl phosphate to ornithine is
catalysed by a liver mitochondrial enzyme:
(A) Carbamoyl phosphate synthetase
(B) Ornithine transcarbamoylase
(C) N-acetyl glutamate synthetase
(D) N-acetyl glutamate hydrolase
115. A compound serving a link between citric
acid cycle and urea cycle is
(A) Malate (B) Citrate
(C) Succinate (D) Fumarate
116. The 2 nitrogen atoms in urea are
contributed by
(A) Ammonia and glutamate
(B) Glutamine and glutamate
(C) Ammonia and aspartate
(D) Ammonia and alanine
117. In carcinoid syndrome the argentaffin
tissue of the abdominal cavity overproduce
(A) Serotonin (B) Histamine
(C) Tryptamine (D) Tyrosine
118. Tryptophan could be considered as
precursor of
(A) Melanotonin (B) Thyroid hormones
(C) Melanin (D) Epinephrine
119. Conversion of tyrosine to dihydroxyphenylalanine
is catalysed by tyrosine hydroxylase
which requires
(A) NAD (B) FAD
(C) ATP (D) Tetrahydrobiopterin
120. The rate limiting step in the biosynthesis
of catecholamines is
(A) Decarboxylation of dihydroxyphenylalanine
(B) Hydroxylation of phenylalanine
(C) Hydroxylation of tyrosine
(D) Oxidation of dopamine
121. The enzyme dopamine â-oxidase which
catalyses conversion of dopamine to
norepinephrine requires
(A) Vitamin A (B) Vitamin C
(C) Vitamin E (D) Vitamin B12
122. In humans the sulphur of methionine and
cysteine is excreted mainly as
(A) Ethereal sulphate
(B) Inorganic sulphate
(C) Sulphites
(D) Thioorganic compound
123. Small amount of urinary oxalates is
contributed by the amino acid:
(A) Glycine (B) Tyrosine
(C) Alanine (D) Serine
124. The amino acid which detoxicated benzoic
acid to form hippuric acid is
(A) Glycine (B) Alanine
(C) Serine (D) Glutamic acid
125. The amino acids involved in the synthesis
of creatin are
(A) Arginine, glycine, active methionine
(B) Arginine, alanine, glycine
(C) Glycine, lysine, methionine
(D) Arginine, lysine, methionine
126. Chemical score of egg proteins is considered
to be
(A) 100 (B) 60
(C) 50 (D) 40
127. Chemical score of milk proteins is
(A) 70 (B) 65
(C) 60 (D) 40
128. Chemical score of proteins of bengal gram
is
(A) 70 (B) 60
(C) 44 (D) 42
129. Chemical score of protein gelatin is
(A) 0 (B) 44
(C) 57 (D) 60
130 Chemical score of protein zein is
(A) 0 (B) 57
(C) 60 (D) 70
131. Biological value of egg white protein is
(A) 94 (B) 83
(C) 85 (D) 77
132. Net protein utilisation of egg protein is
(A) 75% (B) 80%
(C) 91% (D) 72%
133. Net protein utilization of milk protein is
(A) 75% (B) 80%
(C) 86% (D) 91%
134. A limiting amino acid is an essential
amino acid
(A) That is most deficient in proteins
(B) That is most excess in proteins
(C) That which increases the growth
(D) That which increases the weight gain
135. The limiting amino acid of rice is
(A) Lysine (B) Tryptophan
(C) Phenylalanine (D) Tyrosine
136. The limiting amino acid of fish proteins is
(A) Tryptophan (B) Cysteine
(C) Lysine (D) Threonine
137. Pulses are deficient in
(A) Lysine (B) Threonine
(C) Methionine (D) Tryptophan
138. A trace element deficient in the milk is
(A) Magnesium (B) Copper
(C) Zinc (D) Chloride
139. A conjugated protein present in the egg
yolk is
(A) Vitellin (B) Livetin
(C) Albuminoids (D) Ovo-mucoid
140. The chief protein of cow’s milk is
(A) Albumin (B) Vitellin
(C) Livetin (D) Casein
141. A water soluble vitamin deficient in egg is
(A) Thiamin (B) Ribofalvin
(C) Ascrobic acid (D) Cobalamin
142. Pulses are rich in
(A) Lysine (B) Methionine
(C) Tryptophan (D) Phenylalanine
143. Milk is deficient in
(A) Vitamin B1 (B) Vitamin B2
(C) Sodium (D) Potassium
144. Milk is deficient in
(A) Calcium (B) Iron
(C) Sodium (D) Potassium
145. When net protein utilization (NPU) is low,
the requirements for proteins are
(A) High (B) Moderate
(C) Low (D) Supplementary
146. Protein content of human milk is about
(A) 1.4% (B) 2.4%
(C) 3.4% (D) 4.4%
147. Protein content of cow’s milk is about
(A) 2.5% (B) 3.5%
(C) 4.5% (D) 5.5%
148. Protein content of soyabean is about
(A) 30% (B) 40%
(C) 50% (D) 60%
149. Lipid content of egg white is
(A) 12% (B) 33%
(C) 10–11% (D) Traces
150. The recommended daily allowance (RDA)
of proteins for an adult man is
(A) 70 gms (B) 50 gms
(C) 40 gms (D) 30 gms
151. The basic amino acids are
(A) Lysine (B) Bile acids
(C) Glycine (D) Alanine
152. The daily caloric requirement for the
normal adult female is about
(A) 1500 (B) 2100
(C) 2500 (D) 2900
153. In the total proteins, the percentage of
albumin is about
(A) 20–40 (B) 30–45
(C) 50–70 (D) 80–90
154. In the total proteins percentage of α1
globulin is about
(A) 0.2–1.2% (B) 1.2–2.0%
(C) 2.4–4.4% (D) 5.0–10.0%
155. In the total proteins the percentage of
γ globulin is about
(A) 2.4–4.4% (B) 10.0–21.0%
(C) 6.1–10.1% (D) 1.2–2.0%
156. Most frequently the normal albumin
globulin ratioratio (A : G) is
(A) 1.0 : 0.8 (B) 1.5 : 1.0
(C) 2.0 : 1.0 (D) 2.4 : 1.0
157. In Thymol turbidity test the protein
involved is mainly
(A) Albumin (B) á1-Globulin
(C) á2-Globulin (D) â Globulin
158. In quaternary structure, subunits are
linked by
(A) Peptide bonds (B) Disulphide bonds
(C) Covalent bonds (D) Non-covalent bonds
159. Molecular weight of human albumin is
about
(A) 156,000 (B) 90,000
(C) 69,000 (D) 54,000
160. At isoelectric pH, an amino acid exists as
(A) Anion (B) Cation
(C) Zwitterion (D) None of these
161. A disulphide bond can be formed
between
(A) Two methionine residues
(B) Two cysteine residues
(C) A methionine and a cysteine residue
(D) All of these
162 A coagulated protein is
(A) Insoluble
(B) Biologically non-functional
(C) Unfolded
(D) All of the above
163. At a pH below the isoelectric point, an
amino acid exists as
(A) Cation
(B) Anion
(C) Zwitterion
(D) Undissociated molecule
164. An amino acid having a hydrophilic side
chain is
(A) Alanine (B) Proline
(C) Methionine (D) Serine
165. An amino acid that does not take part in
á helix formation is
(A) Histidine (B) Tyrosine
(C) Proline (D) Tryptophan
166. A protein rich in cysteine is
(A) Collagen (B) Keratin
(C) Haemoglobin (D) Gelatin
167. Primary structure of proteins can be
determined by the use of
(A) Electrophoresis (B) Chromatography
(C) Ninhydrin (D) Sanger’s reagent
168. Electrostatic bonds can be formed between
the side chains of
(A) Alanine and leucine
(B) Leucine and valine
(C) Asparate and glutamate
(D) Lysine and aspartate
169. Sanger’s reagent contains
(A) Phenylisothiocyanate
(B) Dansyl chloride
(C) 1-Fluoro-2, 4-dinitrobenzene
(D) Ninhydrin
170. The most abundant protein in mammals is
(A) Albumin (B) Haemoglobin
(C) Collagen (D) Elastin
171. Folding of newly synthesized proteins is
accelerated by
(A) Protein disulphide isomerase
(B) Prolyl cis-trans isomerase
(C) Chaperonins
(D) All of these
172. Primary structure of a protein is formed by
(A) Hydrogen bonds (B) Peptide bonds
(C) Disulphide bonds (D) All of these
173. á-Helix is formed by
(A) Hydrogen bonds
(B) Hydrophobic bonds
(C) Electrostatic bonds
(D) Disulphide bonds
174. Glutelins are present in
(A) Milk (B) Eggs
(C) Meat (D) Cereals
175. Aromatic amino acids can be detected by
(A) Sakaguchi reaction
(B) Millon-Nasse reaction
(C) Hopkins-Cole reaction
(D) Xanthoproteic reaction
176. Two amino groups are present in
(A) Leucine (B) Glutamate
(C) Lysine (D) Threonine
177. During denaturation of proteins, all of the
following are disrupted except
(A) Primary structure (B) Secondary structure
(C) Tertiary structure (D) Quaternary structure
178. All the following are branched chain
amino acids except
(A) Isoleucine (B) Alanine
(C) Leucine (D) Valine
179. An –OH group is present in the side chain of
(A) Serine (B) Arginine
(C) Lysine (D) Proline
180. Edman’s reagent contains
(A) Phenylisothiocyanate
(B) 1-Fluoro-2, 4-dinitrobenzene
(C) Dansyl Chloride
(D) tBOC azide
181. Edman’s reaction can be used to
(A) Determine the number of tyrosine residues in
a protein
(B) Determine the number of aromatic amino acid
residues in a protein
(C) Determine the amino acid sequence of a
protein
(D) Hydrolyse the peptide bonds in a protein
182. Inherited deficiency of â−glucosidase causes
(A) Tay-Sachs disease
(B) Metachromatic leukodystrophy
(C) Gaucher’s disease
(D) Multiple sclerosis
183. Tay-Sachs disease results from inherited
deficiency of
(A) Arylsulphatase A
(B) Hexosaminidase A
(C) Sphingomyelinase
(D) Ceramidase
184. The largest alpolipoprotein is
(A) Apo E (B) Apo B-48
(C) Apo B-100 (D) Apo A-I
185. Apolipoprotein B-100 is synthesised in
(A) Adipose tissue (B) Liver
(C) Intestine (D) Liver and intestine
186. Apolipoprotein B-48 is synthesized in
(A) Adipose tissue (B) Liver
(C) Intestine (D) Liver and intestine
187. Apolipoproteins A-I and A-II are present
in
(A) LDL only
(B) LDL and VLDL
(C) HDL only
(D) HDL and chylomicrons
188. Apolipoprotein B-48 is present in
(A) Chylomicrons (B) VLDL
(C) LDL (D) HDL
189. Apolipoprotein B-100 is present in
(A) Chylomicrons (B) VLDL only
(C) LDL only (D) VLDL and LDL
190. Apolipoproteins C-I, C-II and C-III are
present in
(A) Chylomicrons (B) VLDL
(C) HDL (D) All of these
191. Apolipoprotiens C-I, C-II and C-III are
present in all of the following except
(A) Chylomicrons (B) VLDL
(C) LDL (D) HDL
192. Apolipoprotein A-I acts as
(A) Enzyme activator (B) Ligand for receptor
(C) Both (A) and (B) (D) None of these
193. Apolipoprotien B-100 acts as
(A) Enzyme activator (B) Ligand for receptor
(C) Both (A) and (B) (D) None of these
194. Apolipoprotein C-II is an activator of
(A) Lecithin cholesterola acyl transferase
(B) Phospholipase C
(C) Extrahepatic lipoprotein lipase
(D) Hepatic lipoprotein lipase
195. Nascent chylomicron receives apolipoproteins
C and E from
(A) VLDL remnant (B) VLDL
(C) LDL (D) HDL
196. Terminal transferase
(A) Removes nucleotides from 3’ end
(B) Adds nucleotides at 3’ end
(C) Removes nucleotides from 3’end
(D) Adds nucleotides at 3’end
197. S1 nuclease hydrolyses
(A) DNA of somatic cells
(B) DNA of sperms
(C) Any double stranded DNA
(D) Any single stranded DNA
198. Positive nitrogen balance is seen in
(A) Starvation
(B) Wasting diseases
(C) Growing age
(D) Intestinal malabsorption
199. Alanine can be synthesized from
(A) Glutamate and á-ketoglutarate
(B) Pyruvate and glutamate
(C) Pyruvate and á-ketoglutarate
(D) Asparate and á-ketoglutarate
200. All of the following are required for
synthesis of alanine except
(A) Pyruvate (B) á-ketoglutarate
(C) Glutamate (D) Pyridoxal phosphate
201. All of the following statements about
aspartate are true except
(A) It is non-essential amino acid
(B) It is a dicarboxylic amino acid
(C) It can be synthesized from pyruvate and
glutamate
(D) It can be converted into asparagine
202. Glycine can be synthesized from
(A) Serine (B) Choline
(C) Betaine (D) All of these
203. All of the following are required for
synthesis of glutamine except
(A) Glutamate
(B) Ammonia
(C) Pyridoxal phosphate
(D) ATP
204. A coenzyme required for the synthesis of
glycine from serine is
(A) ATP
(B) Pyridoxal phosphate
(C) Tetrahydrofolate
(D) NAD
205. All of the following statements about
proline are true except
(A) It is an imino acid
(B) It can be synthesized from glutamate
(C) It can be catabolised to glutamate
(D) Free proline can be hydroxylated to
hydroxyproline
206. A protein rich in hydroxyproline is
(A) Prolamin (B) Procollagen
(C) Collagen (D) Proinsulin
207. All the following statement about
hydroxyproline are true except
(A) There is no codon for hydroxyproline
(B) It is present in large amounts in collagen
(C) Free proline cannot be hydroxylated to
hydroxyproline
(D) Hydroxylation of proline residues is catalysed
by a dioxygenase
208. All of the following are required for
hydroxylation of proline residues except
(A) Ascorbic acid (B) Glutamate
(C) Ferrous ions (D) Molecular oxygen
209. Cysteine can be synthesized from
methionine and
(A) Serine (B) Homoserine
(C) Homocysteine (D) Threonine
210. Methionine is synthesized in human body
from
(A) Cysteine and homoserine
(B) Homocysteine and serine
(C) Cysteine and serine
(D) None of these
211. Hydroxylation of phenylalanine requires
all of the following except
(A) Phenylalanine hydroxylase
(B) Tetrahydrobiopterin
(C) NADH
(D) Molecular oxygen
212. Non-Protein amino acids are
(A) Ornithine
(B) â-alanine
(C) ã-amino butyric acid
(D) All of these
213. The amino acid that undergoes oxidative
deamination at significant rate is
(A) Alanine (B) Aspartate
(C) Glutamate (D) Glutamine
214. Allosteric inhibitor of glutamate dehydrogenase
is
(A) ATP (B) ADP
(C) AMP (D) GMP
215. Allsoteric activator of glutamate dehydrogenase
is
(A) ATP (B) GTP
(C) ADP and GDP (D) AMP and GMP
216. Free ammonia is released during
(A) Oxidative deamination of glutamate
(B) Catabolism of purines
(C) Catabolism of pyrimidines
(D) All of these
217. An organ which is extremely sensitive to
ammonia toxicity is
(A) Liver (B) Brain
(C) Kidney (D) Heart
218. Ammonia is transported from muscles to
liver mainly in the form of
(A) Free ammonia (B) Glutamine
(C) Asparagine (C) Alanine
219. The major site of urea synthesis is
(A) Brain (B) Kidneys
(C) Liver (D) Muscles
220. Carbamoyl phosphate required for urea
synthesis is formed in
(A) Cytosol (B) Mitochondria
(C) Both (A) and (B) (D) None of these
221. Cytosolic and mitochondrial carbamoyl
phosphate synthetase have the following
similarity:
(A) Both use ammonia as a substance
(B) Both provide carbamoyl phosphate for urea
synthesis
(C) Both require N-acetylglutamate as an
activator
(D) Both are allosteric enzymes
222. The following enzyme of urea cycle is
present in cytosol:
(A) Argininosuccinic acid synthetase
(B) Argininosuccinase
(C) Arginase
(D) All of these
223. ATP is required in following reactions of
urea cycle:
(A) Synthesis of carbamoyl phosphate and
citrulline
(B) Synthesis of citrulline and argininosuccinate
(C) Synthesis of argininosuccinate and arginine
(D) Synthesis of carbamoyl phosphate and
argininosuccinate
224. Daily excretion of nitrogen by an adult
man is about
(A) 15–20 mg (B) 1.5–2 gm
(C) 5–10 gm (D) 15–20 gm
225. Maple syrup urine diseases is an inborn
error of metabolism of
(A) Sulphur-containing amino acids
(B) Aromatic amino acids
(C) Branched chain amino acids
(D) Dicarboxylic amino acids
226. Cystinuria results from inability to
(A) Metabolise cysteine
(B) Convert cystine into cysteine
(C) Incorporate cysteine into proteins
(D) Reabsorb cystine in renal tubules
227. The defective enzyme in histidinemia is
(A) Histidine carboxylase
(B) Histidine decarboxylase
(C) Histidase
(D) Histidine oxidase
228. All the following statements about
phenylketonuria are correct except
(A) Phenylalanine cannot be converted into
tyrosine
(B) Urinary excretion of phenylpyruvate and
phenyllactate is increased
(C) It can be controlled by giving a lowphenylalanine
diet
(D) It leads to decreased synthesis of thyroid
hormones, catecholamines and melanin
229. All the following statements about
albinism are correct except
(A) Tyrosine hydroxylase (tyrosinase) is absent or
deficient in melanocytes
(B) Skin is hypopigmented
(C) It results in mental retardation
(D) Eyes are hypopigmented
230. Glycine is not required for the formation
of
(A) Taurocholic acid (B) Creatine
(C) Purines (D) Pyrimidines
231. Histamine is formed from histidine by
(A) Deamination (B) Dehydrogenation
(C) Decarboxylation (D) Carboxylation
232. DOPA is an intermediate in the synthesis
of
(A) Thyroid hormones
(B) Catecholamines
(C) Melanin
(D) Catecholamines and melanin
233. All the following statements about pepsin
are correct except
(A) It is smaller than pepsinogen
(B) It is formed by the action of HCl on its precursor
(C) Its optimum pH is 1.0–2.0
(D) It hydrolyses the C-terminal and N-terminal
peptide bonds of proteins
234. Pancreatic juice contains the precursors of
all of the following except
(A) Trypsin (B) Chymotrypsin
(C) Carboxypeptidase (D) Aminopeptidase
235. The only correct statement about chymotrypsin
is
(A) It is formed from trypsin
(B) Carboxypeptidase converts trypsin into
chymotrypsin
(C) Its optimum pH is around 7
(D) It hydrolyses peptide bonds involving basic
amino acids
236. The portion of the antigen molecule which
is recognized by antibody is known as
(A) Hapten (B) Epitope
(C) Complement (D) Variable region
237. All the following statements about
haptens are true except
(A) They have high molecular weights
(B) They cannot elicit an immune response by
themselves
(C) When combined with some other large
molecule, they can elicit an immune response
(D) Once an immune response develops, the free
hapten can be recognized by the antibody
238. Antigens and haptens have the following
similarity:
(A) They have high molecular weights
(B) They can elicit immune response by themselves
(C) They can elicit an immune response only in
association with some other large molecule
(D) Once an immune response develops, free
antigen and free hapten can be recognized
by the antibody
239. The minimum number of polypeptide
chains in an immunoglobulin is
(A) Two (B) Four
(C) Five (D) Six
240. Light chains of immunoglobulins are of
following types:
(A) Alpha and kappa (B) Alpha and gamma
(C) Lambda and delta(D) Kappa and lambda
241 Immunoglobulins are classified on the
basis of
(A) Type of light chains
(B) Type of heavy chains
(C) Types of light and heavy chains
(D) Molecular weight
242. The molecular weight of light chains is
(A) 10,000–15,000 (B) 20,000–25,000
(C) 25,000–50,000 (D) 50,000–75,000
243. The molecular weight of heavy chains is
(A) 20,000–25,000 (B) 25,000–50,000
(C) 50,000–70,000 (D) 70,000–1,00,000
244. Secretory component is present in
(A) IgA (B) IgG
(C) IgM (D) All of these
245. The variable region of light chains is the
(A) N-terminal quarter (B) N-terminal half
(C) C-terminal quarter (D) C-terminal half
246. The variable region of light chain is the
(A) N-terminal quarter
(B) N-terminal half
(C) C-terminal quarter
(D) C-terminal half
247. The variable region of light chains has
(A) One hypervariable region
(B) Two hypervariable regions
(C) Three hypervariable regions
(D) Four hypervariable regions
248. The variable region of heavy chains has
(A) One hypervariable region
(B) Two hypervariable regions
(C) Three hypervariable regions
(D) Four hypervariable regions
249. The most abundant immunoglobulin in
plasma is
(A) IgA (B) IgG
(C) IgM (D) IgD
250. The largest immunoglobulin is
(A) IgA (B) IgG
(C) IgM (D) IgD
251. The plasma concentration of IgA is
(A) 1–5 mg/dl (B) 40–200 mg/dl
(C) 60–500 mg/dl (D) 700–1,500 mg/dl
252. An immunoglobulin found in exocrine
secretions is
(A) IgA (B) IgG
(C) IgM (D) IgE
253. Allergic reactions are mediated by
(A) IgA (B) IgG
(C) IgD (D) IgE
254. An immunoglobulin which can cross the
placental barrier is
(A) IgA (B) IgM
(C) IgD (D) None of these
255. IgM possesses
(A) Two light chains and two heavy chains
(B) Four light chains and four heavy chains
(C) Six light chains and six heavy chains
(D) Ten light chains and ten heavy chains
256. The immunoglobulin having the longest
half-life is
(A) IgA (B) IgG
(C) IgM (D) IgE
257. The half-life of IgG is
(A) 2–3 days (B) 5–6 days
(C) 8–10 days (D) 20–25 days
258. Recognition of antigen is the function of
(A) Variable region of light chains
(B) Variable regions of light and heavy chains
(C) Constant region of heavy chains
(D) Constant regions of light and heavy chains
259. The effector function of antibody is
performed by
(A) Variable region of light chains
(B) Constant region of heavy chains
(C) Variable regions of light and heavy chains
(D) Constant regions of light and heavy chains
260. Complement system can be activated by
binding of antigen to
(A) IgA (B) IgD
(C) IgE (D) IgM
261. C1 component of classical complement
pathway is made up of
(A) Complements 1q and 1r
(B) Complements 1q and 1s
(C) Complements 1r and 1s
(D) Complements 1q, 1r and 1s
262. The components of complement system
are activated by
(A) Microsomal hydroxylation
(B) Phosphorylation
(C) Glycosylation
(D) Proteloysis
263. The component system forms a membrane
attack complex made up of
(A) Complements 1q, 1r and 1s
(B) Complements 1, 2, 3 and 4
(C) Complements 5b, 6, 7 and 8
(D) Factors B and D
264. Factors B and D are required in
(A) The classical pathway of complement fixation
(B) The alternate complement pathway
(C) Both (A) and (B)
(D) None of these
265. The alternate complement pathway
doesn’t involve
(A) Antigen-antibody complex
(B) Complement 3
(C) Factors B and D
(D) Membrane attack unit
266. Antibody diversity arises from
(A) Gene amplification
(B) Gene re-arrangement
(C) Alternative splicing
(D) All of these
267. A light chain gene is constructed from the
following segments:
(A) Variable and constant segments
(B) Variable, joining and constant segments
(C) Variable, diversity and constant segments
(D) Variable, joining, diversity and constant
segments
268. In metabolic point of view, amino acids
are classified as
(A) Glycogenic
(B) Ketogenic
(C) Glycogenic or Ketogenic
(D) All of these
269. Diversity segments are present in
(A) Light chain genes
(B) Heavy chain genes
(C) Light and heavy chain genes
(D) None of these
270. Constant segments of heavy chains are
of
(A) Five types (B) Six types
(C) Seven types (D) Eight types
271. Gamma heavy chains are of
(A) Two types (B) Three types
(C) Four types (D) Five types
272. Gamma heavy chains are present in
(A) IgA (B) IgG
(C) IgM (D) IgD
273. Heavy chains in IgD are of following type:
(A) Alpha (B) Gamma
(C) Delta (D) Epsilon
274. On exposure to any antigen, the first
antibody to be formed is of the following
class:
(A) IgA (B) IgG
(C) IgM (D) IgE
275. Constant segment genes of heavy chains
are present in a cluster in which the first
gene on side is
(A) Alpha (B) Gamma
(C) Delta (D) None of these
276. Cell-mediated immunity is the function of
(A) B lymphocytes (B) T lymphocytes
(C) Plasma cells (D) Basophils
277. The most abundant T cells are
(A) Cytotoxic T cells (B) Helper T cells
(C) Suppressor T cells (D) Memory T cells
278. T cells can recognise
(A) Free antigens
(B) Antigens bound to cells
(C) Antigens bound to antibodies
(D) Antigens bound to MHC proteins
279. MHC proteins are unique to
(A) Each cell (B) Each organ
(C) Each individual (D) Each species
280. MHC class I proteins are present on the
surface of
(A) B cells only (B) T cells only
(C) Macrophages only(D) All cells
281. MHC class I proteins, in conjunction with
antigens are recognised by
(A) Cytotoxic T cells (B) Helper T cells
(C) Suppressor T cells (D) Memory T cells
282. MHC class II proteins are present on the
surface of
(A) All cells
(B) B lymphocytes only
(C) Macrophages only
(D) Macrophages and B lymphocytes
283. MHC Class II proteins, in conjunction with
antigens, are recognised by
(A) Cytotoxic T cells
(B) Helper T cells
(C) Suppressor T cells
(D) Memory T cells
284. CD 8 is a transmembrane glycoprotein
present in
(A) Cytotoxic T cells
(B) Helper T cells
(C) Suppressor T cells
(D) Memory T cells
285. CD 4 is a transmembrane glycoprotein
present in
(A) Cytotoxic T cells (B) Helper T cells
(C) Suppressor T cells (D) Memory T cells
286. CD 3 complex and p 56lck proteins are
present in
(A) Cytotoxic T cells (B) Helper T cells
(C) Both (A) and (B) (D) None of these
287. Cytotoxic T cells release
(A) Perforins
(B) Interleukins
(C) Colony stimulating factors
(D) Tumour necrosis factor
288. Helper T cells release
(A) Interleukins
(B) Colony stimulating factors
(C) Tumour necrosis factor
(D) All of these
289. MHC Class III proteins include
(A) Immunoglobulins
(B) Components of complement system
(C) T cells receptors
(D) CD4 and CD8 proteins
290. Human immunodeficiency virus destroys
(A) Cytotoxic T cells (B) Helper T cells
(C) B cells (D) Plasma cells
291. In allergic diseases, the concentration of
the following is increased in plasma:
(A) IgA (B) IgG
(C) IgD (D) IgE
292. IgE has a tendency to attach to
(A) Basophils (B) Mast cells
(C) Both (A) and (B) (D) None of these
293. Reaginic antibody is
(A) IgA (B) IgG
(C) IgD (D) IgE
294. Active immunity can be produced by
administration of
(A) Killed bacteria or viruses
(B) Live attenuated bacteria or viruses
(C) Toxoids
(D) All of these
295. Passive immunity can be produced by
administration of
(A) Pure antigens
(B) Immunoglobulins
(C) Toxoids
(D) Killed bacteria or viruses
296. Helper T cells release all the following
except
(A) Interleukins
(B) Colony stimulating factors
(C) Perforins
(D) Tumour necrosis factor
297. IgG cleaved by papain into
(A) Two light and two heavy chains
(B) Two Fab and one Fc fragments
(C) Two pairs of one light and one heavy chain
each
(D) One Fab and two Fc fragments
298. Bence-Jones protein is
(A) An immunoglobulin
(B) A dimer of heavy chains
(C) A dimer of light chains
(D) A dimer of one heavy and one light chains
299. Bence-Jones proteins possess all the
following properties except
(A) They are dimers of light chains
(B) Their amino acids sequences are identical
(C) Their N-terminal halves have variable amino
acid sequences
(D) Their C-terminal halves have constant amino
acid sequences
300. A Zwitterion is
(A) Positive ion (B) Negative ion
(C) Both (A) and (C) (D) None of these
301. After accounting for SDA, the net gain of
energy from 25 gm of proteins is about
(A) 70 kcal (B) 100 kcal
(C) 130 kcal (D) 200 kcal
302. After accounting for SDA, the net gain of
energy from 25 gm of carbohydrates is
about
(A) 70 kcal (B) 95 kcal
(C) 100 kcal (D) 105 kcal
303. After accounting for SDA, the net gain of
energy from 100 gm of fat is about
(A) 600 kcal (B) 780 kcal
(C) 900 kcal (D) 1020 kcal
304. If proteins, carbohydrates and fats are
consumed together:
(A) The total SDA is the sum of individual SDAs
of proteins, carbohydrates and fats
(B) The total SDA is more than the sum of
individual SDAs of proteins, carbohydrates
and fats
(C) Carbohydrates and fats lower the SDA of
proteins
(D) Proteins raise the SDA of carbohydrates and
fats
305. After calculating the energy requirement
of a person:
(A) 10% kcal are subtracted on account of SDA
(B) 10% kcal are added on account of SDA
(C) 20% kcal are subtracted on account of SDA
(D) 20% kcal are subtracted on account of SDA
306. The recommended energy intake for an
adult sedentary Indian man is
(A) 1,900 kcal/day (B) 2,400 kcal/day
(C) 2,700 kcal/day (D) 3,000 kcal/day
307. The recommended energy intake for an
adult sedentary Indian woman is
(A) 1,900 kcal/day (B) 2,200 kcal/day
(C) 2,400 kcal/day (D) 2,700 kcal/day
308. During pregnancy, the following should
be added to the calculated energy
requirement:
(A) 300 kcal/day (B) 500 kcal/day
(C) 700 kcal/day (D) 900 kcal/day
309. During first six months of lactation, the
following increment in energy intake is
recommended:
(A) 200 kcal/day (B) 300 kcal/day
(C) 550 kcal/day (D) 1,000 kcal/day
310. The proximate principles of diet are
(A) Vitamins and minerals
(B) Proteins
(C) Carbohydrates and fats
(D) Carbohydrates, fats and proteins
311. The limiting amino acid in wheat is
(A) Leucine (B) Lysine
(C) Cysteine (D) Methionine
312. The limiting amino acid in pulses is
(A) Leucine (B) Lysine
(C) Tryptophan (D) Methionine
313. Maize is poor in
(A) Lysine
(B) Methionine
(C) Tryptophan
(D) Lysine and tryptophan
314. The percentage of ingested protein/
nitrogen absorbed into blood stream is
known as
(A) Net protein utilisation
(B) Protein efficiency ratio
(C) Digestibility coefficient
(D) Biological value of protein
315. Biological value of a protein is
(A) The percentage of ingested protein/nitrogen
absorbed into circulation
(B) The percentage of ingested protein/nitrogen
in the body
(C) The percentage of ingested protein utilised
for protein synthesis in the body
(D) The gain in body weight (gm) per gm of
protein ingested
316. Net protein utilisation depends upon
(A) Protein efficiency ratio
(B) Digestibility coefficient
(C) Digestibility coefficient and protein efficiency
ratio
(D) Digestibility coefficient and biological value
317. The gain in body weight (gm) per gm of
protein ingested is known as
(A) Net protein utilisation
(B) Protein efficiency ratio
(C) Digestibility coefficient
(D) Biological value of protein
318. The following is considered as reference
standard for comparing the nutritional
quality of proteins:
(A) Milk proteins (B) Egg proteins
(C) Meat proteins (D) Fish proteins
319. Biological value of egg proteins is about
(A) 70 % (B) 80 %
(C) 86 % (D) 94 %
320. The following has the highest protein
efficiency ratio:
(A) Milk proteins (B) Egg proteins
(C) Meat proteins (D) Fish proteins
321. The following has the lowest protein
efficiency ratio:
(A) Maize proteins (B) Wheat proteins
(C) Milk proteins (D) Rice proteins
322. Protein content of egg is about
(A) 10% (B) 13%
(C) 16% (D) 20%
323. Protein content of meat is about
(A) 10% (B) 13%
(C) 16% (D) 20%
324. Protein content of rice is about
(A) 7% (B) 12%
(C) 15% (D) 20%
325. The calorific value of wheat is about
(A) 2.5 kcal/gm (B) 3.5 kcal/gm
(C) 4.5 kcal/gm (D) 5.5 kcal/gm
326. For vegetarians, pulses are an important
source of
(A) Carbohydrates (B) Proteins
(C) Fat (D) Iron
327. The amino acids present in pulses can
supplement the limiting amino acids of
(A) Cereals (B) Milk
(C) Fish (D) Nuts and beans
328. Milk is a good source of
(A) Proteins, calcium and iron
(B) Proteins, calcium and ascorbic acid
(C) Proteins, lactose and retinol
(D) Proteins, lactose and essential fatty acids
329. Milk is a good source of all of the following
except
(A) Essential amino acids
(B) Vitamin C
(C) Galactose
(D) Calcium and phosphorous
330. Milk is poor in
(A) Cholesterol (B) Retinol
(C) Calcium (D) Iron
331. Egg is rich in all of the following except
(A) Cholesterol (B) Saturated fatty acids
(C) Ascorbic acid (D) Calcium
332. A phosphoprotein present in egg is
(A) Casein (B) Albumin
(C) Ovoglobulin (D) Ovovitellin
333. Consumption of raw eggs can cause
deficiency of
(A) Calcium (B) Lipoic acid
(C) Biotin (D) Vitamin A
334. Egg is poor in
(A) Essential amino acids
(B) Carbohydrates
(C) Avidin
(D) Biotin
335. Cholesterol is present in all the following
except
(A) Milk (B) Fish
(C) Egg white (D) Egg yolk
336. Meat is rich in all of the following except
(A) Iron (B) Fluorine
(C) Copper (D) Zinc
337. Kwashiorkor occurs when the diet is
severely deficient in
(A) Iron (B) Calories
(C) Proteins (D) Essential fatty acids
338. Clinical features of Kwashiorkor include
all of the following except
(A) Mental retardation (B) Muscle wasting
(C) Oedema (D) Anaemia
339. Kwashiorkor usually occurs in
(A) The post-weaning period
(B) Pregnancy
(C) Lactation
(D) Old age
340. Marasmus occurs from deficient intake of
(A) Essential amino acids
(B) Essential fatty acids
(C) Calories
(D) Zinc
341. Marasmus differs from Kwashiorkor in
the which of these following respect
(A) Mental retardation occurs in kwashiorkor but
not in marasmus
(B) Growth is retarded in kwashiorkor but not in
marasmus
(C) Muscle wasting occurs in marasmus but not
kwashiorkor
(D) Subcutaneous fat disappears in marasmus
but not in kwashiorkor
342. Energy reserves of an average well-fed
adult man are about
(A) 50,000 kcal (B) 100,000 kcal
(C) 200,000 kcal (D) 300,000 kcal
343. During starvation, the first reserve
nutrient to be depleted is
(A) Glycogen (B) Proteins
(C) Triglycerides (D) Cholesterol
344. Synthesis of the following enzymes is
increased during starvation.
(A) Digestive enzymes
(B) Gluconeogenic enzymes
(C) Urea cycle enzymes
(D) Glucokinase
345. In hypoparathyroidism
(A) Plasma calcium and inorganic phosphorous
are low
(B) Plasma calcium and inorganic phosphorous
are high
(C) Plasma calcium is low and inorganic
phosphorous high
(D) Plasma calcium is high and inorganic
phosphorous low
346. The number of amino acid residues in
calcitonin in
(A) 9 (B) 32
(C) 51 (D) 84
347. Calcitonin is synthesised in
(A) Parathyroid glands
(B) Thyroid gland
(C) Pars intermedia of pituitary
(D) Adrenal cortex
348. Plasma calcium is lowered by
(A) Parathormone (B) Calcitonin
(C) Aldosterone (D) Deoxycorticosterone
349. á Cells of Islets of Langerhans secrete
(A) Insulin (B) Glucagon
(C) Somatostatin (D) Cholecystokinin
350. A/G ratio is
(A) Strength of proteins
(B) ratio of serum proteins
(C) ratio of ceruloplasmin
(D) None of these
351. Insulin is made up of
(A) A single polypeptide chain having 51 amino
acid residues
(B) A single polypeptide chain having 84 amino
acid residues
(C) A-chain having 21 and B-chain having 30
amino acid residues
(D) A-chain having 30 and B-chain having 21
amino acid residues
352. The number of amino acid residues in preproinsulin
is
(A) 51 (B) 84
(C) 109 (D) 119
353. Pre-proinsulin contains a signal sequence
having
(A) 9 amino acid residues
(B) 19 amino acid residues
(C) 27 amino acid residues
(D) 33 amino acid residues
354. The number of intra-chain disulphide
bonds in pro-insulin:
(A) One (B) Two
(C) Three (D) Four
355. Pentagastrin is a
(A) Naturally occurring form of gastrin
(B) Inactive metabolite of gastrin
(C) Active metabolite of gastrin
(D) Synthetic form of gastrin
356. Secretion of gastrin is evoked by
(A) Entry of food into stomach
(B) Vagal stimulation
(C) Lower aliphatic alcohols
(D) All of these
357. Gastrin stimulates
(A) Gastric motility (B) Gastric secretion
(C) Both (A) and (B) (D) None of these
358. Secretin is made up of
(A) 17 amino acids (B) 27 amino acids
(C) 37 amino acids (D) 47 amino acids
359. Secretin causes all of the following except
(A) Secretion of pancreatic juice
(B) Secretion of bile
(C) Inhibition of gastric secretion
(D) Stimulation of intestinal motility
360. All of the following statements about
cholecystokinin pancreozymin are true
except
(A) It is secreted by mucosa of small intestine
(B) It stimulates secretion of pancreatic juice rich
in enzymes
(C) It stimulates contraction of gall bladder
(D) It inhibits gastric motility
361. All of the following statements about
pancreatic somatostain are true except
(A) It is secreted by ä cells of islets of Langerhans
(B) It stimulates the secretion of gastrin
(C) It inhibits the secretion of secretin
(D) It inhibits the secretion of cholecystokininpancreozymin
362. Histidine is converted into histamine by
(A) Carboxylation (B) Decarboxylation
(C) Methylation (D) Hydroxylation
363. Histamine is synthesised in
(A) Brain (B) Mast cells
(C) Basophils (D) All of these
364. Histamine causes all the following except
(A) Stimulation of gastric secretion
(B) Vasoconstriction
(C) Pruritus
(D) Increase in capillary permeability
365. H2-receptors are blocked by
(A) Diphenhydramine (B) Mepayramine
(C) Pyrilamine (D) Cimetidine
366. Serotonin is synthesised from
(A) Serine (B) Phenylalanine
(C) Tyrosine (D) Tryptophan
367. All the following statements about
serotonin are true except
(A) It causes vasolidatation
(B) It causes bronchoconstriction
(C) It is metabolized by monoamine oxidase
(D) Its metabolite is 5-hydroxyindole acetic acid
368. All the following statements about
angiotensin are true except
(A) Its precursor is an á2-globulin
(B) Its active form is an octapeptide
(C) It is a vasodilator
(D) It increases the secretion of aldosterone
369. Methyl dopa decreases blood pressure by
(A) Inhibiting the synthesis of catecholamines
(B) Antagonising the action of aldosterone
(C) Stimulating the release of renin
(D) Inhibiting the breakdown of angiotensin
370. Binding of gamma-aminobutyric acid to
its receptors in brain increases the
permeability of cell membrane to
(A) Cl– (B) Na+
(C) K+ (D) Ca++
371. Binding of acetylcholine to its receptors
increases the permeability of cell
membrane to
(A) Ca++ (B) Na+
(C) K+ (D) Na+ and K+
372. All of the following are glycoproteins
except
(A) Collagen (B) Albumin
(C) Transferrin (D) IgM
373. Sialic acids are present in
(A) Proteoglycans (B) Glycoproteins
(C) Both (A) and (B) (D) None of these
374. Hyaluronidase hydrolyses
(A) Hyaluronic acid
(B) Chondroitin sulphate
(C) Heparin
(D) Hyaluronic acid and chondroitin sulphate
375. The most abundant protein in bones is
(A) Collagen type I
(B) Collagen type II
(C) Collagen type III
(D) Non-collagen proteins
376. The most abundant collagen in cartilages
is
(A) Type I (B) Type II
(C) Type III (D) Type IV
377. Collagen and elastin have the following
similarity:
(A) Both are triple helices
(B) Both have hydroxyproline residues
(C) Both have hydrolysine residues
(D) Both are glycoproteins
378. Abnormal collagen structure is seen in all
of the following except
(A) I-cell disease
(B) Osteogenesis imperfecta
(C) Menke’s disease
(D) Ehlers-Danlos sydrome
379. I-cell disease results from absence of the
following from lysosomal enzymes:
(A) Signal sequence
(B) Mannose-6-phosphate
(C) Sialic acid
(D) A serine residue
380. In I-cell disease, lysosomal enzymes
(A) Are not synthesised
(B) Are inactive
(C) Lack signal sequence
(D) Cannot reach lysosomes
381. Renal glycosuria occurs due to
(A) Increased filtration of glucose in glomeruli
(B) Increased secretion of glucose by renal
tubular cells
(C) Decreased reabsorption of glucose by renal
tubular cells
(D) Increased conversion of glycogen into glucose
in tubular cells
382. Haematuria can occur in
(A) Haemolytic anaemia
(B) Mismatched blood transfusion
(C) Yellow fever
(D) Stone in urinary tract
383. Haematuria can occur in all of the following
except
(A) Acute glomerulonephritis
(B) Cancer of urinary tract
(C) Stone in urinary tract
(D) Mismatched blood transfusion
384. Chyluria can be detected by addition of
the following to the urine:
(A) Sulphosalicylic acid (B) Nitric acid
(C) Acetic anhydride (D) Chloroform
385. Normal range of serum urea is
(A) 0.6–1.5 mg/dl (B) 9–11 mg/dl
(C) 20–45 mg/dl (D) 60–100 mg/dl
386. Normal range of serum creatinine is
(A) 0.6–1.5 mg/dl (B) 9–11 mg/dl
(C) 20–45 mg/dl (D) 60–100 mg/dl
387. Standard urea clearance is
(A) 54 ml/min (B) 75 ml/min
(C) 110 ml/min (D) 130 ml/min
388. Maximum urea clearance is
(A) 54 ml/min (B) 75 ml/min
(C) 110 ml/min (D) 130 ml/min
389. Average creatinine clearance in an adult
man is about
(A) 54 ml/min (B) 75 ml/min
(C) 110 ml/min (D) 130 ml/min
390. Inulin clearance in an average adult man
is about
(A) 54 ml/min (B) 75 ml/min
(C) 110 ml/min (D) 130 ml/min
Q391. Among the following, a test of tubular
function is
(A) Creatinine clearance
(B) Inulin clearance
(C) PAH clearance
(D) PSP excretion test
392. A simple way to assess tubular function
is to withhold food and water for 12
hours and, then, measure
(A) Serum urea
(B) Serum creatinine
(C) Urine output in one hour
(D) Specific gravity of urine
393. Among the following, the most sensitive
indicator of glomerular function is
(A) Serum urea
(B) Serum creatinine
(C) Urea clearance
(D) Creatinine clearance
394. All the following statements about inulin
are correct except
(A) It is completely non-toxic
(B) It is completely filtered by glomeruli
(C) It is not reabsorbed by tubular cells
(D) It is secreted by tubular cells
395. Non-protein nitrogenous substances in
blood include all of the following except
(A) Urea (B) Uric acid
(C) Creatinine (D) Inositol
396. Non-protein nitrogenous substances in
blood are raised in
(A) Starvation
(B) Liver damage
(C) Renal failure
(D) All of these
397. Creatinine clearance is deceased in
(A) Acute tubular necrosis
(B) Acute glomerulonephritis
(C) Hypertension
(D) Myopathies
398. Serum amylase is increased in
(A) Acute parotitis (B) Acute pancreatitis
(C) Pancreatic cancer (D) All of these
399. Maximum rise in serum amylase occurs in
(A) Acute parotitis
(B) Acute pancreatitis
(C) Chronic pancreatitis
(D) Pancreatic cancer
400. Serum lipase is increased in
(A) Acute parotitis (B) Acute pancreatitis
(C) Infective hepatitis (D) Biliary obstruction
401. Which one of the following metabolites
is not directly produced in the hexose
monophosphate pathway?
(A) Fructose-6-phosphate
(B) Dihydroxy acetone phosphate
(C) CO2
(D) Erythrose-4-phosphate
402. Which one of the following statements
concerning glucose-6-phosphate dehydrogenase
deficiency is correct?
(A) Young R.B.Cs, particularly reticulocytes,
contain the highest enzyme activity cells show
less enzyme activity
(B) Glucose-6-P Dehydroglucose deficiency
leads to disfuction of many tissues
(C) G-6-p Dehydroglucose deficiency is due to a
single deletion of a large sequence of DNA
in the G-6-PD gene
(D) G-6-PD deficiency is precipitated by ingestion
of drugs such as aspirin
403. The phenomenon of inhibition of glycolysis
by O2 is termed as
(A) Red drop (B) Pasteur effect
(C) Michaelis effect (D) Fischer’s effect
404. Seratonin is derived in the body from the
following amino acid:
(A) Phenylalanine (B) Histidine
(C) Tryptophan (D) Serine
405. Which amino acid is a lipotropic factor?
(A) Lysine (B) Leucine
(C) Tryptophan (D) Methionine
406. Which among the following is a nutritionally
essential amino acid for man ?
(A) Alanine (B) Glycine
(C) Tyrosine (D) Tryptophan
407. The essential amino acids
(A) Must be supplied in the diet because the
organism has lost the capacity to aminate the
corresponding ketoacids
(B) Must be supplied in the diet because the
human has an impaired ability to synthesize
the carbon chain of the corresponding ketoacids
(C) Are identical in all species studied
(D) Are defined as those amino acids which
cannot be synthesized by the organism at a
rate adequate to meet metabolic requirements
408. Which among the following is an essential
amino acid?
(A) Cysteine (B) Leucine
(C) Tyrosine (D) Aspartic acid
409. Which among the following is a basic
amino acid?
(A) Aspargine (B) Arginine
(C) Proline (D) Alanine
410. This amino acid cannot have optical
isomers:
(A) Alanine (B) Histidine
(C) Threonine (D) Glycine
411. The amino acid which contains a
guanidine group is
(A) Histidine (B) Arginine
(C) Citrulline (D) Ornithine
412. GABA(gama amino butyric acid) is
(A) Post-synaptic excitatory transmitter
(B) Post-synaptic inhibitor transmitter
(C) activator of glia-cell function
(D) inhibitor of glia-cell function
413. Sulphur-containing amino acid is
(A) Glutathione (B) Chondroitin sulphate
(C) Homocysteine (D) Tryptophan
414. The useful reagent for detection of amino
acids is
(A) Molisch reagent
(B) Dichlorophenol Indophenol
(C) Ninhydrin
(D) Biuret
415. The amino acid which contains an indole
group is
(A) Histidine (B) Arginine
(C) Glycine (D) Tryptophan
416. Sakaguchi reaction is answered by
(A) Lysine
(B) Ornithine
(C) Arginine
(D) Arginino succinic acid
417. The pH of an amino acid depends
(A) Optical rotation (B) Dissociation constant
(C) Diffusion coefficient(D) Chain length
418. When amino acids are treated with neutral
formaldehyde, the pH of the mixture
(A) Is not altered
(B) Increases
(C) Decreases
(D) First increases then decreases
419. Which among the following has an
imidazole group?
(A) Histidine (B) Tryptophan
(C) Proline (D) Hydroxy proline
420. The amino acid exist as Zwitter ions when
they are in
(A) solid state (B) acidic solution
(C) alkaline solution (D) neutral solution
421. Plasma proteins are isolated by
(A) Salting out (B) Electrophoresis
(C) Flourimetry (D) Both (A) and (B)
422. After digestion amino acids
(A) Are absorbed into portal circulation
(B) Are absorbed into lymph
(C) Are excreted to the extent of 50%
(D) Converted into glucose in the intestine
423. Cysteine has the formula:
(A) CH3SH
(B) H2N—CH2—COOH
(C) HS—CH2—CH(NH2)—COOH
(D) S—CH2—CH(NH2)—COOH
|
S—CH2—CH(NH2)—COOH
424. The compound having the formula
H2N—CO—NH—CH2—CH2—CH2—CH— COOH is
|
NH2
(A) Lysine (B) Glutamine
(C) Serine (D) Citrulline
425. An amino acid which contains a disulphide
bond is
(A) Lysine (B) Methionine
(C) Homocysteine (D) Cystine
426. One of the following has a phenolic group:
(A) Histidine (B) Hydroxy lysine
(C) Seratonine (D) Hydroxy proline
427. An amino acid not containing the usual—
COOH group is
(A) Alanine (B) Tryptophan
(C) Methionine (D) Taurine
428. Branched chain amino acids are
(A) Cysteine and cystine
(B) Tyrosine and Tryptophan
(C) Glycine and Serine
(D) Valine, Leucine and Isoleucine
429. A Zwitter ion is one which has in aqueous
solution:
(A) One positive charge and one negative charge
(B) Two positive charges and one negative charge
(C) Two negative charges and one positive
charge
(D) No electrical charges at all
430. The amino acid which gives yellow colour
with Ninhydrin in paper chromatography
is
(A) Tyrosine (B) Proline
(C) Tryptophan (D) Alanine
431. Hydroxylation of Proline and Lysine in a
protein is effected by
(A) Vitamin B1 (B) Vitamin B2
(C) Vitamin B6 (D) Vitamin C
432. Millon’s test is for identification of
(A) Tyrosine (B) Tryptophan
(C) Proline (D) Arginine
433. Hopkins-Cole test is for identification of
(A) Tyrosine (B) Tryptophan
(C) Arginine (D) Cysteine
434. Collagen is very rich in
(A) Glycine (B) Serine
(C) Aspartic acid (D) Glutamic acid
435. All amino acids are optically active except
(A) Glycine (B) Serine
(C) Threonine (D) Tryptophan
436. Out of 200 different amino acids form in
nature the number of amino acids present
in protein:
(A) 20 (B) 25
(C) 40 (D) 35
437. Enzyme catalyzed hydrolysis of proteins
produces amino acids of the form:
(A) D (B) L
(C) DL (D) All of these
438. The ionizable groups of amino acids are
at least.
(A) 1 (B) 2
(C) 3 (D) 4
439. The neutral amino acid is
(A) Lysine (B) Proline
(C) Leucine (D) Histidine
440. The amino acid containing hydroxyl
group:
(A) Alanine (B) Isoleucine
(C) Arginine (D) Threonine
441. The sulphur containing amino acid:
(A) Homoserine (B) Serine
(C) Methionine (D) Valine
442. The basic amino acid:
(A) Glycine (B) Leucine
(C) Histidine (D) Proline
443. The amino acid which synthesizes many
hormones:
(A) Valine (B) Phenyl alanine
(C) Alanine (D) Histidine
444. Amino acids are insoluble in
(A) Acetic acid (B) Chloroform
(C) Ethanol (D) Benzene
445. The major end product of protein nitrogen
metabolism in man is
(A) Glycine (B) Uric acid
(C) Urea (D) NH3
446. An amino acid not involved in urea cycle
is
(A) Arginine (B) Histidine
(C) Ornithine (D) Citrulline
447. NH3 is detoxified in brain chiefly as
(A) Urea (B) Uric acid
(C) Creatinine (D) Glutamine
448. In humans, NH3 is detoxified in liver as
(A) Creatinine (B) Uric acid
(C) Urea (D) Uronic acid
449. The body protein after eighteen years
(A) Remains unchanged
(B) Is decomposed only slightly at intervals of one
month
(C) Is in a constant state of flux
(D) Is used only for energy requirement
450. The only known physiological methylating
agents in the animal organism are
(A) Choline and betaine
(B) Choline and ä-adenosyl methionine
(C) Betaine and ä-adenyosyl methionine
(D) Dimehtyl glycine and betaine
451. In the synthesis of 1 molecule of urea in
the Kreb’s Hanseleit cycle, the number of
ATPs required is
(A) 1 (B) 2
(C) 3 (D) 4
452. For biosynthesis of proteins
(A) Amino acids only are required
(B) Amino acids and nucleic acids only are
required
(C) Amino acid, nucleic acids and ATP only are
required
(D) Amino acids, nucleic acids, ATP, GTP,
enzymes and activators are required
453. Transmethylation of guanido acetic acid
gives
(A) Creatine phosphate
(B) Creatinine
(C) Choline
(D) n-methyl nicotinamide
454. The 2 energy rich compounds needed for
protein biosynthesis are
(A) ATP and GTP (B) ATP and UTP
(C) ATP and CTP (D) ATP and TTP
455. The following ketoacid is involved in
fixing dietary NH3 into amino acid:
(A) Pyruvate (B) Oxalo acetate
(C) Oxalo succinate (D) á-keto glutarate
456. The metabolite which sustains urea cycle
is
(A) Ornithine
(B) Citrulline
(C) Carbamoyl phosphate
(D) n-acetyl glutamate
457. Tetra hydroglolate can be freed from N5
methyl tetrahydrofolate only by
(A) Nor epinephrine (B) Ethanol amine
(C) Nicotinamide (D) Vitamin B12
458. Neogenesis of methyl group is
(A) The availability of methyl group form ä
adenosyl methionine
(B) The availability of methyl group from betaine
(C) Interaction between N5 N10 methylene tetra
hydrofolate with a NAD+ dependent
reductase
(D) Availability of methyl group from methyl B12
459. More creatinine is excreted by
(A) Adult males (B) Adult females
(C) Children (D) Pregnant women
460. A growing peptide in a ribosome can not
be shifted to the adjacent ribosome
because
(A) It is firmly attached
(B) It will get the amino acid cleaved
(C) The gap between the ribosomes is too big for
a shift
(D) The adjacent ribosomes have different
composition
461. The first amino acid incorporated in a
polypeptide in a ribosome of a human is
(A) N formyl methionine (B) Methionine
(C) Phenyl alanine (D) Hydroxy lysine
462. The first amino acid incorporated in a
polypeptide in a ribosome of a bacterium
is
(A) N formyl methionine (B) Methionine
(C) Alamine (D) Glycine
463. The integrator between the TCA cycle and
urea cycle is
(A) Fumarate (B) Malate
(C) Pyruvate (D) Citrate
464. Bence jones proteinurial characterized by
(A) Non-heat coagulability
(B) Heat coagulability at 100°C
(C) Heat coagulability at 45 to 60°C
(D) Precipitation at 25°C
465. Bence Jones proteins may be excreted in
urine of patients suffering from
(A) Tuberculosis (B) Diabetes mellitus
(C) Multiple myeloma (D) Hyperthyroidism
466. Xanthuric acid is an abnormal metabolite
of
(A) Xanthine (B) Uric acid
(C) Tyrosine (D) Tryptophan
467. Two nitrogen atoms of Urea in the urea
cycle come from
(A) NH3
(B) One from NH3 and one from aspartate
(C) One from NH3 and one from glutamate
(D) One from NH3 and one from alanine
468. Pyruvic acid can be obtained by transamination
of alanine with
(A) á- keto glutaric acid
(B) Acetoacetic acid
(C) â−OH butyric acid
(D) Phosphoenol Pyruvic acid
469. In the synthesis of 1 molecule of urea in
the Kreb’s Henseleit cycle the number of
AMPs formed is
(A) 1 (B) 2
(C) 3 (D) 4
470. Formation of melanin from tyrosine
requires the action of
(A) Dopa decarboxylation
(B) Diamine oxidase
(C) Peroxidase
(D) Tyrosinase
471. In one of the following the quality of the
protein synthesized is affected:
(A) Diabetes mellitus (B) Gont
(C) Multiple myeloma (D) Primaquine sensitivity
472. Citrulline is an intermediate of
(A) TCA cycle (B) Urea cycle
(C) Pentose cycle (D) Calvin cycle
473. The semialdehydes are formed under the
action of enzymes characterised as
(A) Aldolases
(B) Peptidyl lysyl oxidases
(C) Collagenases
(D) Elastases
474. Which of the following statement about
the peptide bond is true?
(A) It is a carbon-carbon bond
(B) It has cis hydrogen and oxygen groups
(C) It is planar
(D) It has rotational freedom
475. Isoenzymes for a given reaction
(A) Have different spedificities
(B) Have identical affinities for the same substrate
(C) Exhibit different electrophoretic motilities
(D) Contain similar ratios of different polypeptide
chains
476. The highest concentration of cystine can
be found in
(A) Melanin (B) Chondroitin sulphate
(C) Myosin (D) Keratin
477. One round of Edman degradation of the
peptide: H2N— Gly—Arg—Lys—Phe—
Asp— COOH would result in which of the
following structures or their phenyl isothiocyanate
derivatives?
(A) H2N—Gly—Arg—COOH + H2N—Lys—
Phe— Asp—COOH
(B) H2N—Gly—Arg—Lys—Phe—COOH + Asp
(C) H2N—Arg—Lys—Phe—Asp—COOH + Gly
(D) H2N—Gly—Arg—Lys—COOH + H2N—Phe
—Asp—COOH
478. Which of the following techniques is used
to separate proteins based upon differences
in their mass?
(A) Isoelectric focusing
(B) Dialysis
(C) SDS-gel Electrophoresis
(D) Western blotting
479. The greatest buffering capacity at
physiologic pH would be provided by a
protein rich in which of the following
amino acids ?
(A) Lysine (B) Histidine
(C) Aspartic acid (D) Valine
480. Which one of the amino acids could serve
as the best buffer at pH 7?
(A) Glutamic acid (B) Arginine
(C) Valine (D) Histidine
481. Which one of the following statements
concerning glutamine is correct?
(A) Contains three tetratable groups
(B) Is classified as an acidic amino acid
(C) Contains an amide group
(D) Migrates to the cathode during electrophoresis
at pH 7.0
482. One of the given example is an amino
acid:
(A) Oh-Lysine (B) Protein
(C) Leucine (D) Serine
483. The lone pair of electrons at one of the
ring nitrogens in the given amino acid
makes a potential ligand, which is
important in binding the iron atoms in
hemoglobin:
(A) Tryptophan (B) Threonine
(C) Histidine (D) Serine
484. The amino acid which is not optically
active is
(A) Alanine (B) Glycine
(C) Glutamine (D) Lysine
485. Optically active compounds are capable of
(A) Different reactions
(B) Rotating plane of polarized light
(C) Showing same chemical properties
(D) None of these
486. The reference compound for absolute configuration
of optically active compound is
(A) Alanine (B) Lactic acid
(C) Glyceraldehyde (D) Dihydroxy acetone
487. All the standard amino acids except the
following have one chiral ‘c’ atom:
(A) Threonine, Isoleucine
(B) Isoleucine, Alanine
(C) Threonine, Alanine
(D) Alanine, Glutamine
488. The role of complement proteins:
(A) Defense
(B) Helps immunity of the body
(C) Not predicatable
(D) None of these
489. Optical isomers that are mirror images
and non superimposable are called
(A) Diastereomers (B) Euantiomers
(C) dl isomers (D) Stereomers
490. Living cells have the unique ability to
synthesize only _________ the form of
optical isomer due to _________.
(A) ‘d’ form, stereospecific enzymes
(B) ‘l’ form stereospecific enzymes
(C) ‘d’ form, DNA
(D) ‘L’ form, DNA
491. Isoelectric pH of an amino acid is that pH
at which it has a
(A) Positive charge (B) Negative charge
(C) No net charge (D) All of these
492. Albuminoids are similar to
(A) Albumin (B) Globulin
(C) Both A and B (D) None of these
493. Abnormal chain of amino acids in sickle
cells anaemia is
(A) Alpha chain (B) Beta chain
(C) Gama chain (D) Delta chain
494. In prehepatic jaundice, protein flocculation
test is
(A) Normal/weekly positive
(B) Usually positive
(C) Negative
(D) None of these
495. Side chains of all amino acids contain
aromatic rings except
(A) Pheynl alanine (B) Alanine
(C) Tyrosine (D) Tryptophan
496. In Nitroprusside test, amino acid cystein
produces
(A) Blue colour complex
(B) Red colour
(C) Yellow colour
(D) Purple colour
497. Bonds that are formed between two
cysteine residues is
(A) Disulphide (B) Peptide
(C) Electrostatic (D) Hydrophobic
498. The acid amide of Aspartic acid is
(A) Glutamine (B) Arginine
(C) Aspargine (D) Ornithine
499. It is the only amino acid having an
ionizing ‘R’ group with a pK’ near 7 and
is important in the active site of some
enzymes:
(A) Arginine (B) Cystein
(C) Cystine (D) Histidine
500. Hemoglobin has a high content of this
amino acid:
(A) Proline (B) Leucine
(C) Arginine (D) Histicline
501. A hexa peptide with 5 aspartic acid would
have a net charge at pH 7:
(A) Neutral (B) Positive
(C) Negative (D) Not predictable
502. In the genetic disorder of cystinuria, the
patient excretes large quantities of
cystine in their urine and its low solubility
causes crystalline cystine to precipitate as
stones in kidneys. The remedy involves
ingesting Na HCO3. Reaction of this
treatment is
(A) NaHCO2 combines with cystine
(B) NaHCO3 raises the pH above the isoelectric
point of cystine
(C) NaHCO3 prevents stone formation by
hydrolysis of cystine to cysteine
(D) None of these
503. In the following reaction, Alanine acts as a
3 3
3 3
+ → +
H H
| |
H N – C –COO—— H N – C –COOH
| |
CH CH
(A) Acid (B) Base
(C) Zwitter ion (D) None of these
504. Amino acids excepting histidine are not
good buffering agents in cell because
(A) They exist as zwitter ions
(B) Their pk and not in the physiological pH of a
cell
(C) Only Histidine has pk of its R group at 6.0
unlike the others which have at a different pH
(D) None of these
505. At neutral pH Alanine has the following
structure:
(A) − − 2
3
H
H N C COOH
CH
(B) + 3 − −
3
H
H N C COO
CH
(C) 2 − −
3
H
H N C COO
CH
(D) + 2 − −
3
H
H N C COO
CH
506. The amino acids in which the R groups
have a net positive charge at pH 7.0 are
(A) Lysine, Arginine, Histidine
(B) Lysine, Aspargine
(C) Histidine, Aspargine
(D) Glutamine, Arginine
507. Apolipoproteins are
(A) AI (B) AI1
(C) C1 (D) All of these
508. The amino acid which has a pK near 4 and
thus is negatively charged at pH 7 is
(A) Alanine (B) Glutamic acid
(C) Glutamine (D) Aspargine
509. The side chain of which of the following
amino acid contain sulphur atom?
(A) Methionine (B) Threonine
(C) Leucine (D) Tryptophan
510. Which of the followings gives a positive
test for Ninhydrin?
(A) Reducing sugars (B) Triglycerides
(C) Alpha aminoacids (D) Esterified Fats
511. In glutathione (a tripeptide) is present
apart from Glutamic acid and cysteine:
(A) Serine (B) Glycine
(C) Leucine (D) Phenyl alanine
512. 2-Amino 3-OH propanoic acid is
(A) Glycine (B) Alanine
(C) Valine (D) Serine
513. All amino acids have one asymmetric
carbon atom, except
(A) Arginine (B) Aspargine
(C) Histidine (D) Glycine
514. Number of amino acids present in the
plant, animal and microbial proteins:
(A) 20 (B) 80
(C) 150 (D) 200
515. Immunoglobulins are characterized by their
(A) Heavy chains
(B) Molecular weight
(C) Light chains
(D) Electrophoretic behaviour
516. The bond in proteins that is not hydrolysed
under usual conditions of denaturation:
(A) Hydrophobic bond (B) Hydrogen bond
(C) Disulphide bond (D) Peptide bonds
517. If the amino group and a carboxylic group
of the amino acid are attached to same
carbon atom, the amino acid is called
(A) Alpha (B) Beta
(C) Gamma (D) Delta
518. Zymogen is
(A) An intracellular enzyme
(B) Serum enzyme
(C) A complete extracellular enzyme
(D) An inactivated enzyme
519. SGOT level in a adult is
(A) 5–40 units/dl (B) 1–4 units/dl
(C) 5–15 units/dl (D) 50–100 units/dl
520. Activity of ceruloplasmin shown in vitro:
(A) Reductase (B) Hydrolase
(C) Ligase (D) Oxidase
521. Increased serum alanine during fasting is
due to
(A) Breakdown of muscle proteins
(B) Decreased utilization of non essential amino
acids
(C) Leakage of aminoacids to plasma
(D) Impaired renal function
522. The following 4 amino acids are required
for completion of urea cycle except
(A) Aspartic acid (B) Arginine
(C) Ornithine (D) Glycine
523. Number of amino acids present in the
dietary proteins:
(A) 22 (B) 23
(C) 20 (D) 19
524. Urea synthesis takes place in
(A) Blood (B) Liver
(C) Kidney (D) Heart
525. All followings are ketogenic aminoacids
except
(A) Leucine (B) Isoleucine
(C) Phenyl alanine (D) Glycine
526. The amino acid containing an indole ring:
(A) Tryptophan (B) Arginine
(C) Threonine (D) Phenylalanine
527. Histidine is converted to histamine
through the process of
(A) Transamination
(B) Decarboxylation
(C) Oxidative deamination
(D) Urea cycle
528. Physiologically active configuration of
amino acids:
(A) L
(B) D
(C) For some amino acids it is either of two
(D) Neither L nor D
529. Cystine is synthesized from
(A) Cysteine (B) Methionine
(C) Arginine (D) Leucine
530. The major constituent of the proteins of
hair and keratin of skin:
(A) Arginine (B) Cysteine
(C) Glycine (D) Arginine
531. NH3 is removed from brain mainly by
(A) Creatinine formation
(B) Uric acid production
(C) Urea formation
(D) Glutamine formation
532. Mechanism by which NH3 is removed from
the kidneys is
(A) Urea formation
(B) Uric acid formation
(C) Creatinine formation
(D) None of these
533. Low density plasma proteins are rich in
(A) Chylomicrons (B) Cholesterol
(C) Triglycerides (D) Phospholipids
534. Transcortins are
(A) Mucoproteins (B) Glycoproteins
(C) Metalloproteins (D) Lipoproteins
535. Proteins that carries Iron into different
tissues is
(A) Ceruloplasmin (B) Trans cortin
(C) Mucoproteins (D) Glycoproteins
536. Naturally occurring amino acids have
(A) L-Configuration (B) D-Configuration
(C) DL-Configuration (D) None of these
537. Abnormal chain of aminoacids in sickle
cell anemia is
(A) â-chain (B) â-chain
(C) ã-chain (D) r-chain
538. A dietary deficiency of tryptophan and
nicotinate leads to
(A) Beri Beri (B) Xerophthalmia
(C) Anemia (D) Pellegra
539. Which one of the following is an essential
amino acid?
(A) Arginine (B) Tyrosine
(C) Phenylalanine (D) Proline
540. One of the following amino acid is solely
ketogenic:
(A) Lysine (B) Alanine
(C) Valine (D) Glutamate
541. Along with CO2, NH3 and ATP, the amino
acid that is needed in urea cycle is
(A) Alanine (B) Isoleucine
(C) Aspartate (D) Glycine
542. Isoelectric pH of an amino acid is that pH
at which it has a
(A) Positive charge (B) Negative charge
(C) No charge (D) None of these
543. Which of the following contributes
nitrogen atoms to both purine and
pyrimidine rings?
(A) Aspartate
(B) Carbamoyl phosphate
(C) CO2
(D) Glutamine
544. Which amino acid is a lipotropic factor?
(A) Lysine (B) Lecuine
(C) Tryptophan (D) Methionine
545. Which of the following protein is rich in
cysteine?
(A) Elastine (B) Collagen
(C) Fibrin (D) Keratin
546. Which amino acid is present at 6th position
of â-chain of Hbs instead of glutamate in
HbA?
(A) Cysteine (B) Valine
(C) Aspartate (D) Glutamate
547. The amino acid which contains an indole
group is
(A) Histidine (B) Arginine
(C) Cystine (D) Tryptophan
548. From two amino acids peptide bond
formation involves removal of one
molecule of
(A) Water (B) Ammonia
(C) Carbondioxide (D) Carboxylic acid
549. Polymers of more than 100 amino acids
are termed
(A) Proteins (B) Polypeptides
(C) Both (A) and (B) (D) None of these
550. The example of globulins:
(A) Leucosin (B) Tuberin
(C) Oryzenin (D) Legunelin
551. The example of scleroproteins:
(A) Glutamin (B) Giladin
(C) Salmine (D) Elastin
552. The example of phosphoprotein:
(A) Mucin (B) Ovovitellin
(C) Ovomucoid (D) Tendomucoid
553. The example of metalloproteins:
(A) Siderophilin (B) OREES mucoid
(C) Elastin (D) All of these
554. The example of chromoprotein:
(A) Salmine (B) Catalase
(C) Zein (D) Gliadin
555. Deamination is ______ of amino group.
(A) Removal (B) Addition
(C) Supplementation (D) None of these
556. Proteins produce polypeptides from
proteins by
(A) Oxidizing (B) Reducing
(C) Hydrolyzing (D) None of these
557. Proteins react with biuret reagent which
is suggestive of 2 or more
(A) Hydrogen bonds (B) Peptide bonds
(C) Disulphide bonds (D) Hydrophobic bonds
558. The disulphide bond is not broken under
the usual conditions of
(A) Filtration (B) Reduction
(C) Oxidation (D) Denaturation
559. Insulin is oxidized to separate the protein
molecule into its constituent polypeptide
chains without affecting the other part of
the molecule by the use of
(A) Performic acid (B) Oxalic acid
(C) Citric acid (D) Malic acid
560. Each hydrogen bond is quite
(A) Weak (B) Strong
(C) Both (A) and (B) (D) None of these
561. A coiled structure in which peptide bonds
are folded in regular manner by
(A) Globular proteins (B) Fibrous proteins
(C) Both (A) and (B) (D) None of these
562. In many proteins the hydrogen bonding
produces a regular coiled arrangement
called
(A) á-helix (B) â-helix
(C) Both (A) and (B) (D) None of these
563. Many globular proteins are stable in
solution although they lack in
(A) Hydrogen bonds (B) Salt bonds
(C) Non-polar bonds (D) Disulphide bonds
564. Each turn of á-helix contains the number
of amino acids
(A) 2.8 (B) 3.2
(C) 3.4 (D) 3.6
565. The distance travelled per turn of á-helix
in nm is
(A) 0.34 (B) 0.44
(C) 0.54 (D) 0.64
566. á-helix is disrupted by certain amino
acids like
(A) Proline (B) Arginine
(C) Histidine (D) Lysine
567. á-helix is stabilized by
(A) Hydrogen bonds (B) Disulphide bonds
(C) Salt bonds (D) Non-polar bonds
568. Foetal haemoglobin contains
(A) Two á and two ã chains
(B) Two â and two ã chains
(C) Both (A) and (B)
(D) None of these
569. When haemoglobin takes up oxygen
there is a change in the structure due to
the moving closer together of
(A) â-chains (B) â-chains
(C) ã-chains (D) á and ã chains
570. The hydrogen bonds in the secondary and
tertiary structure of proteins are directly
attacked by
(A) Salts (B) Alkalies
(C) Detergents (D) All of these
571. The hydrogen bonds between peptide
linkages are interfered by
(A) Guanidine (B) Uric acid
(C) Salicylic acid (D) Oxalic acid
572. The digestability of certain denatured
proteins by proteolytic enzymes
(A) Decreases (B) Increases
(C) Normal (D) None of these
573. The antigenic antibody functions of
proteins by denaturation are frequently
(A) Not changed (B) Changed
(C) Both (A) and (B) (D) None of these
574. In case of severe denaturation of protein,
there is
(A) Reversible denaturation
(B) Moderate reversible denaturation
(C) Irreversible denaturation
(D) None of these
575. When egg albumin is heated till it is
coagulated, the secondary and tertiary
structures of the proteins are completely lost
resulting in a mixture of randomly arranged
(A) Dipeptide chains (B) Tripeptide chains
(C) Polypeptide chains(D) All of these
576. In glycoproteins the carbohydrate is in the
form of disaccharide units, the number of
units are
(A) 50–100 (B) 200–300
(C) 400–500 (D) 600–700
577. The milk protein in the stomach of the
infants is digested by
(A) Pepsin (B) Trypsin
(C) Chymotrypsin (D) Rennin
578. Achylia gastrica is said to be when absence
of
(A) Pepsin only (B) Both pepsin and HCl
(C) HCl only (D) All of these
579. The pH of gastric juice become low in
(A) Hemolytic anemia (B) Pernicious anemia
(C) Both (A) and (B) (D) None of these
580. In small intestine trypsin hydrolyzes
peptide linkages containing
(A) Arginine (B) Histidine
(C) Serine (D) Aspartate
581. Chymotrypsin in the small intestine
hydrolyzes peptide linkages containing
(A) Alanine (B) Pheynl alanine
(C) Valine (D) Methionine
582. Carboxy peptidase B in the small
intestine hydrolyzes peptides containing
(A) Leucine (B) Isoleucine
(C) Arginine (D) Cysteine
583. The transport of amino acids regulated by
active processes of different numbers:
(A) 1 (B) 2
(C) 3 (D) 4
584. The third active process for amino acids
transport involves
(A) Acidic amino acids
(B) Basic amino acids
(C) Neutral amino acids
(D) Sulphur containing amino acids
585. The neutral amino acids for absorption
need
(A) TPP (B) B6 – PO4
(C) NAD+ (D) NADP+
586. If one amino acid is fed excess, the
absorption of another is
(A) Slightly accelerated
(B) Moderately accelerated
(C) Highly accelerated
(D) Retarded
587. Under normal conditions, food proteins
are generally readily digested upto the
present
(A) 67 to 73 (B) 74 to 81
(C) 82 to 89 (D) 90 to 97
588. By overheating the nutritional value of
cereal proteins is
(A) Increased (B) Decreased
(C) Unchanged (D) None of these
589. More than half of the protein of the liver
and intestinal mucosa are broken down
and resynthesised in
(A) 10 days (B) 12 days
(C) 15 days (D) 18 days
590. The half-life of antibody protein is about
(A) 4 weeks (B) 3 weeks
(C) 2 weeks (D) 1 week
591. Protein anabolism is stimulated by
(A) ACTH (B) Testosterone
(C) Glucagon (D) Epinephrine
592. The metabolism of protein is integrated
with that of carbohydrate and fat through
(A) Oxaloacetate (B) Citrate
(C) Isocitrate (D) Malate
593. The building up and breaking down of
protoplasm are concerned with the
metabolism of
(A) Carbohydrate (B) Lipid
(C) Protein (D) Minerals
594. The amino acids abstracted from the liver
are not utilized for repair or special
synthesis but are broken down to
(A) Keto acids (B) Sulphur dioxide
(C) Water (D) Ammonia
595. The unwanted amino acids abstracted
from the tissues are either used up by the
tissue or in the liver converted into
(A) Ammonia (B) Urea
(C) Ammonium salts (D) Uric acid
596. Amino acids provide the nitrogen for the
synthesis of
(A) The bases of the phospholipids
(B) Uric acid
(C) Glycolipids
(D) Chondroitin sulphates
597. The metabolism of all proteins ingested
over and above the essential requirements
is called
(A) Exogenous metabolism
(B) Endogenous metabolism
(C) Both (A) and (B)
(D) None of these
598. Sulphur containing amino acids after
catabolism produces a substance which
is excreted:
(A) SO2 (B) HNO3
(C) H2SO4 (D) H3PO4
599. Ethereal sulphate is synthesized from the
_________ amino acid.
(A) Neutral (B) Acidic
(C) Basic (D) Sulphur containing
600. The amino acids required for creatine
formation:
(A) Glycine (B) Arginine
(C) Methionine (D) All of these
601. In human and other ureotelic organisms,
the end product of amino acid nitrogen
metabolism:
(A) Bile acids (B) Ketone bodies
(C) Urea (D) Barium sulphate
602. The end product of amino acid nitrogen
metabolism in uricotelic organisms
(reptiles and birds) is
(A) Bilirubin (B) Urea
(C) Uric acid (D) Biliverdin
603. The transaminase activity needs the
coenzyme:
(A) ATP (B) B6 – PO4
(C) FAD+ (D) NAD+
604. Transamination is a
(A) Irreversible process(B) Reversible process
(C) Both (A) and (B) (D) None of these
605. Most amino acids are substrates for
transamination except
(A) Alanine (B) Threonine
(C) Serine (D) Valine
606 Oxidative conversion of many amino
acids to their corresponding -ketoacids
occurs in mammalian:
(A) Liver and kidney (B) Adipose tissue
(C) Pancreas (D) Intestine
607. The á-ketoacid is decarboxylated by H2O2
forming a carboxylic acid with one carbon
atom less in the absence of the enzyme:
(A) Catalase (B) Decarboxylase
(C) Deaminase (D) Phosphatase
608. The activity of mammalian L-amino acid
oxidase, an FMN – flavo protein, is quite
(A) Slow (B) Rapid
(C) Both (A) and (B) (D) None of these
609. From dietary protein as well as from the
urea present in fluids secreted into the
gastrointestinal tract intestinal bacteria
produce
(A) Carbondioxide
(B) Ammonia
(C) Ammonium sulphate
(D) Creatine
610. The symptom of ammonia intoxication
includes
(A) Blurring of vision (B) Constipation
(C) Mental confusion (D) Diarrhoea
611. Ammonia intoxication symptoms occur
when brain ammonia levels are
(A) Slightly diminished (B) Highly diminished
(C) Increased (D) All of these
612. Ammonia production by the kidney is
depressed in
(A) Acidosis (B) Alkalosis
(C) Both (A) and (B) (D) None of these
613. Ammonia is excreted as ammonium salts
during metabolic acidosis but the majority
is excreted as
(A) Phosphates (B) Creatine
(C) Uric acid (D) Urea
614. Synthesis of glutamine is accompanied
by the hydrolysis of
(A) ATP (B) ADP
(C) TPP (D) Creatin phosphate
615. In brain, the major metabolism for
removal of ammonia is the formation of
(A) Glutamate (B) Aspartate
(C) Asparagine (D) Glutamine
616. Carbamoyl phosphate synthetase structure
is marked by change in the presence
of
(A) N-Acetyl glutamate
(B) N-Acetyl Aspartate
(C) Neuraminic acid
(D) Oxalate
617. The biosynthesis of Urea occurs mainly in
the Liver:
(A) Cytosol
(B) Microsomes
(C) Nucleus
(D) Mitochondria
618. One mol. of Urea is synthesized at the
expense of the _______ mols. of ATP.
(A) 2 (B) 3
(C) 4 (D) 5
619. Urea biosynthesis occurs mainly in the
liver involving the number of amino acids:
(A) 3 (B) 4
(C) 5 (D) 6
620. The normal daily output of Urea through
urine in grams:
(A) 10 to 20 (B) 15 to 25
(C) 20 to 30 (D) 25 to 35
621. In severe acidosis, the output of urea is
(A) Decreased (B) Slightly increased
(C) Highly increased (D) Moderately increased
622. Uremia occurs in
(A) Cirrhosis of the liver(B) Nephritis
(C) Diabetes mellitus (D) Coronary thrombosis
623. Clinical symptom in urea cycle disorder is
(A) Mental retardation (B) Drowsiness
(C) Diarrhoea (D) Oedema
624. The sparing action of methionine is
(A) Tyrosine (B) Cystine
(C) Arginine (D) Tryptophan
625. NH+
4 aminates glutamate to form
glutamine requiring ATP and
(A) K+ (B) Na+
(C) Ca++ (D) Mg++
626. Glutathione is a
(A) Dipeptide (B) Tripeptide
(C) Polypeptide (D) None of these
627. All following are conjugated proteins
except
(A) Nucleoproteins (B) Proteoses
(C) Metalloproteins (D) Flavoproteins
628. All á-amino acids have one asymmetric
carbon atom except
(A) Arginine (B) Glycine
(C) Aspartic acid (D) Histidine
629. Number of amino acids present in plants,
animals and microbial proteins:
(A) 20 (B) 80
(C) 150 (D) 200
630. Hydrated density of (HD) lipoproteins is
(A) 0.94 gm/ml
(B) 0.94-1.006 gm/ml
(C) 1.006-1.063 gm/ml
(D) 1.063-1.21 gm/l
631. The bond in proteins that is not broken
under usual conditions of denaturation:
(A) Hydrophobic bond (B) Hydrogen bond
(C) Disulphide bond (D) Peptide bonds
632. Plasma proteins act as
(A) Buffers (B) Immunoglobulins
(C) Reserve proteins (D) All of these
633. Group that reacts in the Biuret test:
(A) Peptide (B) Amino group
(C) Carboxylic group (D) Aldehyde group
634. In nitroprusside test, amino acid cysteine
produces a:
(A) Red colour (B) Blue colour
(C) Yellow colour (D) Purple colour
635. Protein present in hemoglobin has the
structure known as
(A) Primary (B) Secondary
(C) Tertiary (D) Quarternary
636. Isoelectric pH of an amino acid is that pH
at which it has a
(A) Positive charge (B) Negative charge
(C) Nil net charge (D) None of these
637. Albuminoids are similar to
(A) Albumin (B) Globulin
(C) Both (A) and (B) (D) None of these
638. Optical isomers of all aminoacids exist
except
(A) Glycine (B) Arginine
(C) Alanine (D) Hydroxy proline
639. Proteins that constitute keratin, collagen
and elastin in body are
(A) Protamines (B) Phosphol proteins
(C) Scleroproteins (D) Metaproteins
640. Systematic name of lysine is
(A) Amino acetic acid
(B) 2,6 diaminohexanoic acid
(C) Aminosuccinic acid
(D) 2-Aminopropanoic acid
641. Side chains of all following amino acids
contain aromatic rings except
(A) Phenyl alanine (B) Alanine
(C) Tyrosine (D) Tryptophan
642. Abnormal chain of amino acids in sickle
cell anaemia is
(A) Alpha chain (B) Beta chain
(C) Delta chain (D) Gama chain
643. Number of chains in globin part of normal
Hb:
(A) 1 (B) 2
(C) 3 (D) 4
644. The PH of albumin is
(A) 3.6 (B) 4.7
(C) 5.0 (D) 6.1
645. Ninhydrin reaction gives a purple colour
and evolves CO2 with
(A) Peptide bonds (B) Histamine
(C) Ergothioneine (D) Aspargine
646. Denaturation of proteins involves
breakdown of
(A) Secondary structure(B) Tertiary structure
(C) Quarternary structure(D) All of these
647. In denaturation of proteins, the bond
which is not broken:
(A) Disulphide bond (B) Peptide bond
(C) Hydrogen bond (D) Ionic bond
648. The purity of an isolated protein can be
tested by employing various methods.
(A) Solubility curve
(B) Molecular weight
(C) Ultra Centrifugation
(D) Immuno Ractivity
(E) All of these
649. More than one break in the line or in saturation
curve indicates the following
quality of protein.
(A) Non homogenity (B) Purity
(C) Homogeneity (D) None of these
650. A sharp moving boundary is obtained
between the pure solvent and solute
containing layer in
(A) Chromatography
(B) Immuno Reactivity
(C) Ultra Centrifugation
(D) Solubility curve
651. The antibodies raised against a pure
protein will show only one sharp spike on
this technique:
(A) Solubility curve
(B) Solvent precipitation
(C) Molecular weight determination
(D) Immuno electrophoresis
652. This technique takes the advantage of the
fact that each protein has different pH at
which it is electrically neutral i.e., its
isoelectric pH:
(A) Isoelectric focussing
(B) Immunoel Ectro Phoresis
(C) Chromatography
(D) HPLC
653. The following technique makes use of the
difference in net charges of proteins at a
given pH:
(A) Thin layer chromatography
(B) Ion exchange chromatography
(C) High performance liquid chromatography
(D) Paper chromatography
654. The ratio of the distance moved by a
compound to the distance moved by the
solvent frent is known as its
(A) PI value (B) Linking number
(C) Rf value (D) Gold number
655. The movement of charged particles
towards one of the electrodes under the
influence of electrical current is
(A) Gel filtration
(B) Molecular sieving
(C) Gas liquid chromatography
(D) Electrophoresis
656. An anion exchange resin linked to
cellulose backbone is
(A) DEAE cellulose (B) CM cellulose
(C) Sephadex (D) None of these
657. A cation exchange resin linked to cellulose
backbone is
(A) CM-cellulose (B) DEAE cellulose
(C) Starch (D) Biogel
658. The sorting out of molecules according to
size and shape may be adapted to protein
purification in this technique:
(A) Adsorption chromatography
(B) Gel filtration chromatography
(C) Paper chromatography
(D) None of these
659. Frequently employed materials for the
adsorption chromatography of proteins
include
(A) High capacity supporting gel
(B) Starch blocks
(C) Calcium phosphate gel alumina gel and
hydroxy apatite
(D) All of these
660. The solubility of most proteins is lowered
at high salt concentrations is called as
(A) Salting in process (B) Salting out process
(C) Isoelectric focussing(D) None of these
661. Phenylalanine, ornithine and methionine
are involved in the biogenesis of
(A) Lysergic acid (B) Reserpine
(C) L-Hyoscyamine (D) Papaverine
662. All the following diuretics inhibit the
carbonic anhydrase except
(A) Acetazolamide (B) Bumetanide
(C) Furosemide (D) Ethacrynic acid
663. Protein is a polymer of
(A) Sugars (B) Phenols
(C) Amino acids (D) Carboxylic acids
664. All the following amino acids are optically
active except
(A) Tryptophane (B) Phenylalanine
(C) Valine (D) Glycine
665. Proteinous substances which catalyze
biochemical reactions are known as
(A) Activators (B) Catalysts
(C) Enzymes (D) Hormones
666. Insulin is a protein which controls
(A) Blood clotting (B) Metabolic pathway
(C) Digestion (D) Kreb’s cycle
667. Proteins which are responsible for defence
mechanism are called
(A) Antimetabolites (B) Antibodies
(C) Antimycins (D) Apoproteins
668. When the net charge on an amino acid is
zero, the pH is maintained as?
(A) 4.5 (B) 11.2
(C) 7.0 (D) 9.1
669. Isoelectric point of amino acids is used for
(A) Crystallisation (B) Precipitation
(C) Solubility (D) Reactivity
670. Xanthoproteic test is positive in proteins
containing
(A) Sulphur amino acids
(B) á-Amino acids
(C) Aromatic amino acids
(D) Aliphatic amino acids
671. All á-amino acids give positive
(A) Million’s test (B) Biurete test
(C) Xanthproteic test (D) Ninhydrine test
672. N-terminal amino acids of a polypeptide
are estimated by
(A) Edmann reaction (B) Sanger’s reagent
(C) Formaldehyde test (D) Ninhydrine reaction
673. Million’s test is positive for
(A) Phenylalanine (B) Glycine
(C) Tyrosine (D) Proline
674. Indole group of tryptophan responses
positively to
(A) Glyoxylic acid (B) Schiff’s reagent
(C) Biuret test (D) Resorcinol test
675. Guanidine group of argentine gives
positive test with
(A) Lead acetate
(B) Sakaguchi reagent
(C) Tricholoroacetic acid
(D) Molisch’s reagent
676. Thiol group of cysteine gives red colour
with
(A) Sodium acetate
(B) Lead acetate
(C) Sodium nitroprusside
(D) Barfoed’s reagent
677. Protein deficiency disease is known as
(A) Cushing’s disease
(B) Fabry’s disease
(C) Parkinson’s disease
(D) Kwashiorkor and marasmus
678. A vegetable source of protein is
(A) Egg plant
(B) Soyabean
(C) Tree of the Heaven
(D) Devil’s dung
679. Oxaloacetate is converted to aspartic acid
by
(A) Reductase (B) Oxidase
(C) Transminase (D) Catalase
680. Deficiency of biotin results in decrease in
(A) Amino acid synthesis
(B) Lipid synthesis
(C) Kidney
(D) Fatty acid synthesis
681. The precursor of bile salts, sex hormones
and vitamin D is
(A) Diosgenin (B) Cholesterol
(C) Campesterol (D) Ergosterol
682 Unsaturated fatty acids is known as
(A) Non-essential fatty acids
(B) Essential fatty acids
(C) Cerebrosides
(D) Phospholipids
683 Biuret test is specific for
(A) Two peptide linkage
(B) Phenolic group
(C) Imidazole ring
(D) None of these
684. Most of calcium is present in bone, but 2%
present in soft tissue and the blood is
called
(A) Calcinated blood (B) Solidified blood
(C) Physiological blood(D) Colloidal blood
685. Calcium present with protein is known as
free while in salt form is called as
(A) Bound (B) Precipitated
(C) Solid (D) Polymorphs
686. The following ions help in enzymatic
transfer of phosphate from ATP to pyruvic
acid:
(A) Sodium (B) Calcium
(C) Magnesium (D) Potassium
687. International enzyme commission classifies
enzymes into
(A) Three classes (B) Six classes
(C) Four classess (D) Ten classes
688. Michaelis – Menten equation is used to
explain the effect of substrate concentration
on
(A) Carbohydrate (B) Enzyme
(C) Lipid (D) Protein
689. The pH at which an enzyme has maximum
activity is known as
(A) Isoelectric pH (B) Optimum pH
(C) Low pH (D) High pH
690. Degradation of proteins to amino acids,
glucose from carbohydrates and fatty
acids from lipids is known as
(A) Anabolism (B) Metabolism
(C) Catabolism (D) Cretinism
691. During glycolysis of glucose the energy
liberated in the absence of oxygen is
known as
(A) Oxygenesis
(B) Glyconeogenesis
(C) Glycogenolysis
(D) Anaerobic fermentation
692. Deficiency of urea cycle enzymes results
into accumulation of citrulline argininosuccinate
arginine in the liver resulting in increasing
concentration of …….. in the blood.
(A) Calcium (B) Sodium
(C) Ammonia (D) Lipid
693. Accumulation of trytophan in blood is
known as
(A) Pompe’s disease (B) Wilson’s disease
(C) Wolman’s disease (D) Hartnup’s disease
694. Lymphocytes are responsible for the formation
of
(A) Serum (B) Plasma
(C) Antibody (D) Calcium
695. Platelets contain an enzyme which has
important role in clotting in blood. This
enzyme is known as
(A) Cholinesterase (B) Transaminase
(C) Decarboxylase (D) Thrombokinase
696. Treatment of pentoses with a concentrated
mineral acid yields a cyclic aldehyde
known as
(A) Pentaldehyde (B) Cyclopental
(C) Hexaldehyde (D) Furfural
697. Isoelectric pH is that pH at which protein
is electrically:
(A) Neutral (B) Anionic
(C) Cationic (D) None of these
698. About 6.25 g of haemoglobin is produced
and destroyed in the body each day and the
total amount of haemoglobin in a normal
healthy 70 kg weighing male adult is
(A) 250 g (B) 150 g
(C) 100 g (D) 70 g
699. Pancreatic juice contains all of the
following except
(A) Trypsinogen (B) Lipase
(C) Cholecystokinin (D) Chymnotrypsinogen
700. The milk protein in the stomach in an adult
is digested by
(A) Pepsin (B) Rennin
(C) HCl (D) Chymotrypsinogen
701. Carboxypeptidase, an enzyme of
pancreatic juice, contains
(A) Mn (B) Zinc
(C) Magnesium (D) Manganese
702. The zymogen from trypsinogen of
pancreatic juice is converted to active
trypsin by
(A) Peisin (B) Enterocrinin
(C) Enterokinase (D) Rennin
703. Inactive zymogens are precursors of all
the following gastrointestinal enzymes
except
(A) Carboxypeptidase (B) Pepsin
(C) Amino peptidase (D) Chymotrypsin
Q `q2qw w2 2q w12704. Rennin acts on casein of milk in infants in
presence of
(A) Mg++ (B) Zn++
(C) Co++ (D) Ca++
705. All the following are true about phenylketonuria
except
(A) Deficiency of phenylalanine hydroxylase
(B) Mental retardation
(C) Increased urinary excretion of p-hydroxyphenyl
pyruvic acid
(D) Decrease serotonin formation
706. Which of the amino acid produces a
vasodilator on decarboxylation?
(A) Glutamin acid (B) Histidine
(C) Ornithine (D) Cysteine
707. Neutral amino acid is
(A) Leucine (B) Lysine
(C) Aspartic acid (D) Histidine
708. The amino acid containing hydroxy group:
(A) Glycine (B) Isoleucine
(C) Arginine (D) Thereonine
709. The amino acid which synthesizes many
hormornes:
(A) Valine (B) Phenylalanine
(C) Alanine (D) Histidine
710. Insulin degradation of disulfide bond
formation is effected by
(A) Pyruvate dehydrogenase
(B) Xylitol reductase
(C) Gutathione reductase
(D) Xanthine oxidase
711. A protein reacts with biuret reagent which
indicates 2 or more
(A) Blood clotting (B) Peptide bond
(C) Disulphide bonds (D) Hydrophobic bonds
712. In many proteins the hydrogen bonding
produces a regular coiled arrangement
which is called as
(A) â-Helix (B) á-Helix
(C) Both (A) and (B) (D) Spiral
713. The milk protein in the stomach of the
infants is digested by
(A) Pepsin (B) Trypsin
(C) Chymotrypsin (D) Rennin
714. Protein anabolism is stimulated by
(A) ACTH (B) Testosterone
(C) Glucagon (D) Epinephrine
715. The number of helices present in a collagen
molecule is
(A) 1 (B) 2
(C) 3 (D) 4
716. Which bond is present in the primary
structure of protein?
(A) Ester (B) Hydrogen
(C) Ionic bond (D) Peptide
717. Sakaguchi reaction is specific for
(A) Guanidine group (B) Phenolic group
(C) Carboxylic group (D) None of these
718. With the exception of glycine all amino
acids found in protein are
(A) Isocitrate dehydrogenase
(B) Fumarase
(C) Succinate thiokinase
(D) ATPase
719 In protein structure the á-helix and â–
pleated sheets are example of
(A) Primary structure (B) Secondary structure
(C) Tertiary structure (D) Quaternary structure
720. An essential amino acid in man is
(A) Proline (B) Threonine
(C) Asparagine (D) Tyrosine
721. An amino acid that does not form an á–
helix is
(A) Asparagine (B) Tyrosine
(C) Tryptophan (D) Proline
722. The protein present in hair is
(A) Elastin (B) Prolamine
(C) Keratin (D) Gliadin
723. Plasma protein can be separated by
(A) Salting out with (NH4)2SO4
(B) Ultracentrifugation
(C) Immuno electrophoresis
(D) All of these
724. RNA does not contain
(A) Uracil
(B) Adenine
(C) Hydroxy methyl cytosine
(D) Phosphate
725. In mammalian cells, ribosomal RNA is
produced mainly in the
(A) Nucleus
(B) Nucleolus
(C) Ribosome
(D) Golgi apparatus
726. Which co-enzyme is not involved in
oxidative decarboxylation of pyruvic
acid?
(A) TPP (B) Mg++
(C) Biotin (D) CoA-SH
727. A polymeric unit of starch which has a
branched structure is
(A) Glucose (B) Amylopectin
(C) Isomaltose (D) Amylose
728 The repeating unit in hyaluronic acid is
(A) Glucuronic acid and Galactosamine
(B) Glucuronic acid are glucosamine
(C) Glucuronic acid and N-acetyl glucosamine
(D) Glucuronic acid and N-acetyl galactosamine
729 The repeating disaccharide unit in
celluslose is
(A) Sucrose (B) Maltose
(C) Dextrose (D) Cellobiose
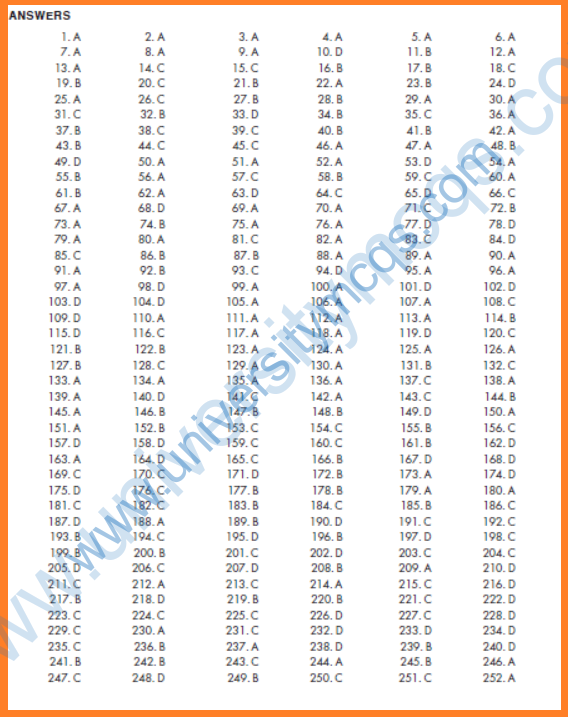
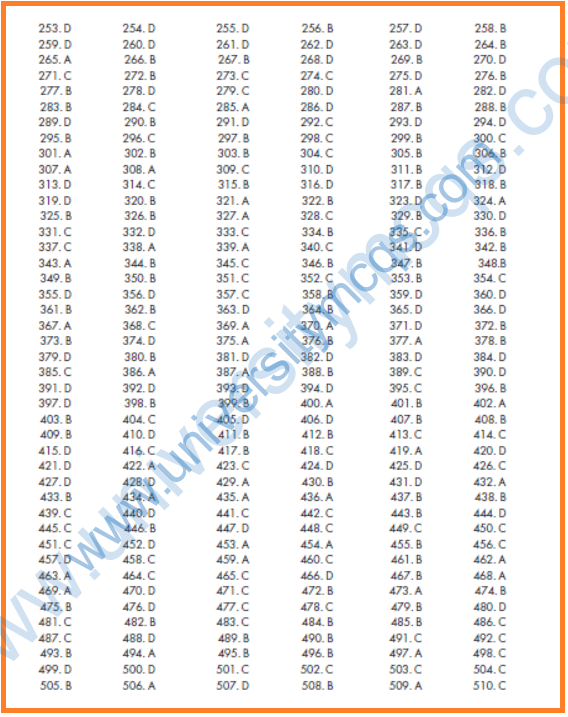
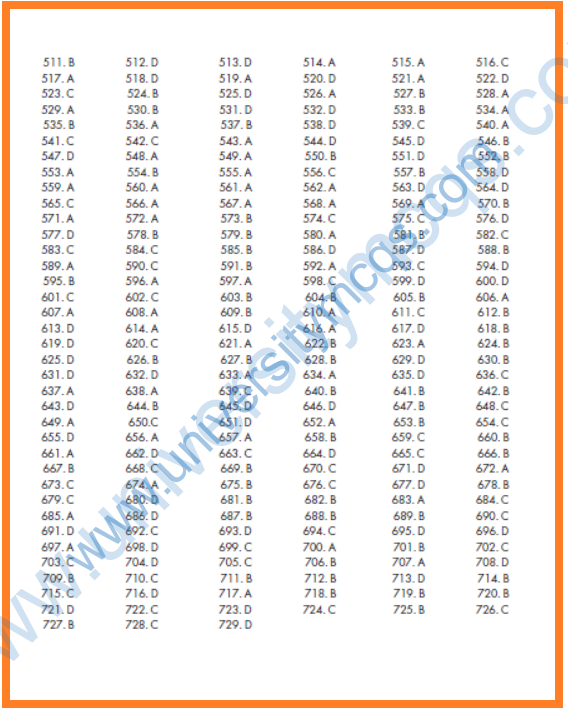
![]()
