Difference between Orbit and Orbital
Before moving on to orbits and orbitals, let’s first discuss Atoms and their basic concepts. An atom is the fundamental building block of matter that makes up a chemical element. Protons and neutrons make up the nucleus of an atom encircled by electrons. The element that an atom represents is determined by the number of protons in its nucleus.
For example, all carbon atoms have six protons in their nucleus, whereas uranium atoms have 92 protons. The fundamental building blocks of chemical composition(molecules) can be created by joining atoms. The configuration of an element’s atoms and how they interact with one another determine the element’s properties.
Orbits and Orbital Theory
The configuration of electrons in an atom and their interactions with the nucleus are described by the orbits and orbitals theory of atoms. It is based on the ideas of quantum mechanics, a fundamental physics theory that describes the behavior of very small-scale particles.
In contrast to how planets circle the Sun, in theory, electrons are not assumed to be in precise/fixed orbits around the nucleus. Atomic orbitals are a series of mathematical operations characterizing the probability of finding the electron at a specific place around the nucleus. In an atom, electrons first occupy orbitals with the lowest energies. For instance, the lone electron in the hydrogen atom is located in the 1s orbital. The higher energy orbitals of the atom are filled when more electrons are added. For lithium electrons, with 3 electrons in orbit, 2 electrons are in 1s orbitals, while the remaining 1 electron resides in higher energy 2s orbital.
The configuration of electrons in an atom’s orbitals dictates its chemical characteristics and interactions with other atoms. The theory of orbits and orbitals describes how atoms and molecules behave and determines the atomic-level characteristics of materials. It has greatly influenced how we perceive the world and has sparked numerous significant technical advancements. Many other theories and postulates describe how atoms behave and how electrons revolve or occupy their position around the nucleus.
Orbits:
In 1913, Neil Bohr gave his atomic theory, according to which electrons can only revolve around certain fixed circular paths with fixed energy and momentum known as Orbits; these circular orbits around the nucleus of an atom are called atomic orbits.
An atomic orbit is an electron’s circular path around the atom’s nucleus. These orbits correspond to specific energy levels, and an electron’s energy is quantized, meaning it can only have certain specific values.
Also, the atomic orbits have fixed energy and angular momentum, indicating that electrons can only move around these circular paths. Atomic orbits are significant because they determine atom properties and behavior in chemical reactions. The structure and behavior of atomic orbits are fundamental concepts in chemistry and physics.
An atomic orbit is an electron’s path around an atom’s nucleus. These orbits correspond to specific energy levels, and an electron’s energy is quantized, meaning it can only have certain specific values. The behavior of an electron in an atom is described in quantum mechanics using wave functions, which can be used to calculate the probability of finding the electron at a given point in space.
On the other hand, an orbital is a mathematical description of an electron’s probability distribution in an atom. It is a region of space around the nucleus where the electron is highly likely to be found. The orbital theory is based mostly on quantum mechanics; after that, many theories have come; but this orbital theory has remained valid to date.
- Atomic orbits are the paths that electrons follow around the nucleus of an atom.
- The energy of an electron in an atomic orbit is quantized, meaning that it can only have certain specific values.
- Other names, such as allowed energy-states or stationary states, are also known as atomic orbits.
- The energy of an atomic orbit is fixed and does not change.
- Atomic orbits are named n1, n2, n3, n4, n5, etc. These integral numbers are called principal Quantum numbers.
Orbitals:
Atomic orbitals are a mathematical representation of an atom’s probability of having an electron at a particular place near its nucleus. They are utilized to predict how atoms will interact with one another to form molecules and characterize the behavior of electrons in atoms. S, P, D, and F represent an atom’s four primary categories of atomic orbitals. Each kind is associated with a certain shape and degree of energy. When compared to the p orbitals, which are shaped like dumbbells, the s orbitals, which are spherical, have lower energy.
In contrast to the p orbitals, which have a more complicated structure, the d orbitals have a more complex shape and greater energy.
In an atom, electrons first occupy orbitals with the lowest energies. The lone electron in the hydrogen atom is located in the 1s orbital. The higher energy orbitals are filled when more electrons are added. The configuration of electrons in an atom’s orbitals defines the atom’s chemical characteristics.
Quantum mechanics, a foundational physics theory that explains the behavior of particles on an extremely small scale, is used to describe atomic orbitals. It allows scientists to make predictions about the behavior of electrons in atoms and molecules and to understand the properties of materials at the atomic level.
- Atomic orbitals are frequently identified by letters and numbers that represent particular characteristics of the orbitals’ associated electrons. A mix of letters and digits is used to identify orbitals. The number denotes the energy level of the orbital, and the letter denotes the kind of orbital (s, p, d, or f). The 1s orbital, for instance, has less energy than the 2s orbital.
- The electrons first occupy the lowest energy orbitals in an atom. As an illustration, the two electrons in the helium atom fill the 1s orbital before any electrons occupy the 2s orbital.
- Two electrons, at most, with opposing spins, can fit into each orbital. Pauli’s exclusion principle is used to describe this. The mathematical functions describing the s, p, d, and f orbitals define their forms. The probability of discovering an electron at a specific position within the nucleus.
- Scientists use the geometries of the s, p, d, and f orbitals to forecast how atoms will interact to form molecules. For instance, a carbon-carbon link can be created when the p orbitals of two carbon atoms overlap and form a chemical connection.
Types of Orbitals:
An orbital is a mathematical function that describes the wave-like behavior of an electron in an atom in chemistry and quantum mechanics. Orbitals are classified according to the shapes they describe and the energies of the electrons they contain.
Orbitals are classified as follows:
1. S-orbitals: These are spherical and have a single lobe. S orbitals are found at the lowest energy level (the 1s orbital) and have the lowest energy. S orbitals, also known as s-type or s-wave orbitals, have a single lobe and are spherical. They are found at the lowest energy level (the 1s orbital) and have the least amount of energy of any orbital. Because the s orbitals are symmetrical around the atom’s nucleus, the probability of finding an electron is equal in all directions. S orbitals are important in determining an atom’s chemical properties. They are also important in determining an atom or molecule’s size and shape. The energy level at which s orbitals are found is used to identify them. The 1s orbital, for example, is the lowest energy s orbital and is found at the first energy level. At the second energy level, the 2s orbital is found, and so on.
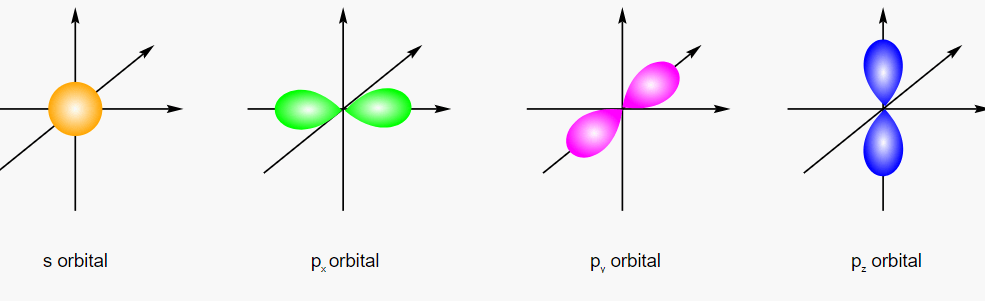
2. P-orbitals: These are shaped like two lobes on opposite sides of the nucleus, with a nodal plane (a plane of zero probability) running through the center. Each energy level has three p orbitals (px, py, and Pz).
3. D-orbitals: They have four lobes and are more complex in shape. Each energy level has five d orbitals (dxy, dyz, dxy, dx2-y2, and dz2).
4. f orbitals: These are more complex, with seven lobes. Each energy level has seven f orbitals (labeled as fx, fy, fz, fxy, fxz, fyz, and fx2-y2).
Each orbital can hold a maximum of two electrons, and electrons in an atom fill the orbitals from lowest to highest energy level.
Difference between Orbits and Orbitals
| Atomic Orbits | Atomic Orbitals |
|---|---|
| These are the path that an electron follows around the nucleus of an atom | A mathematical description of the probability distribution of an electron in an atom. |
| Quantized energy of electrons in orbits. | It is responsible for the behavior of electrons in an atom. |
| Atomic orbits are numbered from n= 1,2,3,4 etc. | In comparison, atomic orbitals are categorized as S- orbital, p-orbital, d-orbital, f- orbital, etc. |
| Neil Bohr gave the concept of Atomic orbits. | Robert Millikan gave the concept of atomic orbitals in 1932. |



